T?rk Toraks Derne?i ast?m tan? ve tedavi rehberi: Anahtar noktalar
F?sun YILDIZ1, ?. K?v?lc?m
O?UZ?LGEN2, Berna DURSUN3, Dil?ad MUNGAN4,
Bilun GEM?C?O?LU5,
Arzu YORGANCIO?LU6 ve T?rk Toraks Derne?i Ast?m ve Allerji ?al??ma
Grubu Ast?m Tan? ve
Tedavi Rehberi Komitesi*
1 Kocaeli ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Kocaeli,
2 Gazi ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Ankara,
3 SB Atat?rk G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi, Ankara,
4 Ankara ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Allerjik Hastal?klar Bilim Dal?, Ankara,
5 ?stanbul ?niversitesi ?stanbul T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, ?stanbul,
6 Celal Bayar ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Manisa.
* ?znur Abado?lu, ?lknur Ba?yi?it, Sevim Bavbek, ?lk? Bay?nd?r, Hasan Bayram, ?smet Bulut, Berrin Ceyhan, Arif ??mr?n, Haluk ?oku?ra?, Elif Da?l?, Berna Dursun, Dane Ediger, M?nevver Erdin?, Feyza Erkan, Bilun Gemicio?lu, Nermin G?ler, A. Fuat Kalyoncu, B?lent Karada?, G?l Karakaya, G?lbin Karako?, Emel Kurt, Zeynep M?s?rl?gil, Dil?ad Mungan, ?. K?v?lc?m O?uz?lgen, Cans?n Sa?kesen, Necla Song?r, E. B?lent ?ekerel, Remziye Tana?, Ayfer Tuncer, Haluk T?rkta?, ?pek T?rkta?, F?sun Y?ld?z, Arzu Yorganc?o?lu, Hasan Y?ksel.
?ZET
T?rk Toraks Derne?i ast?m tan? ve tedavi rehberi: Anahtar noktalar
Ast?m, d?nyada ve ?lkemizde patogenez, tan? ve tedavisinde t?m ilerlemelere ra?men morbiditesi ve maliyeti y?ksek bir hastal?kt?r. Do?ru tan? ve tedavi ile kontrol alt?na al?nabilen bir hastal?k olmas?na ra?men d?nyada ve ?lkemizde belirlenen d???k kontrol oranlar? sadece hastal???n de?i?ken seyrine ve hastalar?n psikososyal kronik hastal?k davran???na ba?lanamaz. Bu ba?lamda, T?rk Toraks Derne?i de en son 2000 y?l?nda yay?nlad??? ?Ast?m Tan? ve Tedavi Rehberi?ni g?ncelleme karar? alm??t?r. ?lkemizin verileri toplanm??, konu ile ilgili e?itimcilerden olu?turulan yazarlar taraf?ndan kan?ta dayal? bilgiler derlenerek haz?rlanm?? ve T?rk Toraks Derne?i Ast?m ve Allerji ?al??ma Grubu taraf?ndan son ?ekli verilerek, dan??man ki?i ve kurumlara sunulmu?tur. Haziran 2009 tarihinde T?rk Toraks Derne?i ?Ast?m Tan? ve Tedavi Rehberi? T?rk?e olarak yay?nlanm??t?r. Bu derlemede ulusal rehberin temel ?zellikleri ve di?erlerinden farklar? ?ngilizce olarak sunulmaktad?r.
Anahtar Kelimeler: Ast?m, tan?, tedavi, rehber.
SUMMARY
Turkish Thoracic Society asthma management and prevention guideline: key points
F?sun YILDIZ1, ?. K?v?lc?m
O?UZ?LGEN2, Berna DURSUN3, Dil?ad MUNGAN4,
Bilun GEM?C?O?LU5,
Arzu YORGANCIO?LU6 and TTS Asthma and Allergy Working Group
Guideline Committee for
Asthma Diagnosis and Treatment*
1 Department of Chest Diseases, Faculty of Medicine, Kocaeli University, Kocaeli, Turkey,
2 Department of Chest Diseases, Faculty of Medicine, Gazi University, Ankara, Turkey,
3 Ataturk Chest Disease and Chest Surgery Training and Research Hospital, Ankara, Turkey,
4 Division of Allergic Diseases, Department of Chest Diseases, Faculty of Medicine, Ankara University,
Ankara, Turkey,
5 Department of Chest Diseases, Faculty of Istanbul Medicine, Istanbul University, Istanbul, Turkey,
6 Department of Chest Diseases, Faculty of Medicine, Celal Bayar University, Manisa, Turkey.
*?znur Abado?lu, ?lknur Ba?yi?it, Sevim Bavbek, ?lk? Bay?nd?r, Hasan Bayram, ?smet Bulut, Berrin Ceyhan, Arif ??mr?n, Haluk ?oku?ra?, Elif Da?l?, Berna Dursun, Dane Ediger, M?nevver Erdin?, Feyza Erkan, Bilun Gemicio?lu, Nermin G?ler, A. Fuat Kalyoncu, B?lent Karada?, G?l Karakaya, G?lbin Karako?, Emel Kurt, Zeynep M?s?rl?gil, Dil?ad Mungan, ?. K?v?lc?m O?uz?lgen, Cans?n Sa?kesen, Necla Song?r, E. B?lent ?ekerel, Remziye Tana?, Ayfer Tuncer, Haluk T?rkta?, ?pek T?rkta?, F?sun Y?ld?z, Arzu Yorganc?o?lu, Hasan Y?ksel.
Asthma still has high morbidity and cost despite all advances in pathogenesis, diagnosis and treatment. Although asthma can be controlled with proper diagnosis and treatment, the low rates of control in our country and in the world can not be attributed to the variable course of the disease and patients? psycho-social behaviours for chronic disease. In this context, Turkish Thoracic Society (TTS) has decided to update Asthma Diagnosis and Management Guide latest published in 2000. National data were collected, compiled and prepared by authors, and final form given by the TTS Asthma and Allergy Study Group, after presenting to consultant individuals and institutions. In June 2009, the National Asthma Management and Prevention Guideline were published in Turkish. In this paper, we aimed to present the national guide in English with its basics and individual differences.
Key Words: Asthma, diagnosis, treatment, guideline.
DEFINITION and EPIDEMIOLOGY
Asthma is a chronic inflammatory disorder of the airways. The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing, particularly at night or in the early morning. These episodes are usually associated with variable airflow obstruction which is often reversible either spontaneously or with treatment (1).
It is estimated that asthma affect 300 million individuals in worldwide. Hundreds of reports on the prevalence of asthma from different populations have shown wide range on asthma prevalence. The global prevalence of asthma ranged from 1% to 18% (1).
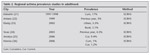
In Turkey, prevalence of asthma has important differences between cities and regions. Asthma prevalence is higher in coastal regions, urban areas, metropolitan cities and at lower socioeconomic conditions (2,3,4,5,6). There is an increase in global prevalence, mortality and morbidity of asthma in last 30 years, but some recent studies has showen that the prevalence of asthma tends to be stabilize or even to decrease (7,8,9,10). The prevalence of asthma both in childhood and adulthood in Turkey are shown in Table 1 and Table 2 (2,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25). Results of national multicentered studies of Turkey are also shown in Table 3 (3,26,27,28).
Social and Economic Burden
Asthma effects the community not only economically but also socially. Asthma is an important cause of absence from school and days lost from work all around the world. Thus, mentioning the economic burden of asthma, it should cover both medical and non-medical costs. Unfortunately, there is lack of data on this issue in Turkey. In a prospective study including adult asthmatics from Ankara, the mean annual total cost of asthma was found as 1467 ? 111.8 USD (29). Another study from Ankara including childhood asthma patients showed that mean annual total cost of asthma is 991.7 ? 73.2 USD (median: 688 USD) (30). A multicentered childhood study from Turkey found that mean annual total cost of asthma is 1597.4 ? 236.2 USD (31). The authors also reported that the annual cost of asthma is associated with unplanned doctor visits, hospitalization, asthma severity and days lost from school. Adulthood asthma study also found similar results (32).
RISK FACTORS
Factors influencing the development and expression of asthma are well known and include both host and environmental factors (1). In our published guide we mostly mentioned about the findings of national studies.
Genetic Factors
Asthma has complex heritable component. Multiple genes have roles in the pathogenesis of asthma (1,33). There are four major areas for the genetic intervention: production of allergen specific IgE antibodies, expression of airway hyperresponsiveness, generation of inflammatory mediators and determination of the ratio between Th1 and Th2 immune responses (1,34).
Obesity
Obesity is another risk factor for asthma. Leptin may involve in airway disfunction and developing of asthma (1,34,35).
Gender
In early childhood male sex is a risk factor for asthma. But as children get older the difference between genders decrease and in adulthood prevalence of asthma is greater in women than men (1,34,35,36).
Allergens
It is well known that both indoor and outdoor allergen exposure can lead increase in asthma symptoms, but their role in the development of asthma is still not clear (1,37,38). Some studies shown that exposure to house dust mite may be a causal factor in the development of asthma (39). It is also shown that exposure to cockroaches is an important cause of sensitization (40,41). Some epidemiologic studies found that early exposure to cats and dogs could protect against sensitization or the development of asthma, but others suggested that those kinds of exposure could increase the risk of sensitization (1). Children grown in rural areas have less prevelance of asthma which could be explained by hygene hypothesis (1,29,42).
Infections
The hygene hypothesis suggests that infections in early life influence the child?s immune system along a ?non-allergic? pathway, leading to a reduced risk of asthma and other allergic diseases (1). However, the interaction between viral infections and atopy is a complex situation.
Occupational Sensitizers
Many substances have been associated with occupational asthma. It is estimated that occupational sensitizers cause approximately 10% of adult asthma cases. Immunmediated occupational asthma with small molecules such as isocyanates has a latency period of months to years. Irritant induced asthma (previoulsy named reactive airways dysfunctional syndrome) occurs with intense exposure to irritants (1,34).
Smoking
Smoking and/or second hand smoke is associated with decline in lung function, increase in symptoms and medication requirements, triggers asthma attacks (14,34). Both prenatal and postnatal exposure to tobacco smoke lead asthma like symptoms in early childhood (17,43). Recently, it is found that 11.4% of asthmatic patients were smokers, which is lower than the percentage of smokers in the general population of Turkey (44%) (44).
Outdoor/Indoor Air Pollution
Although the role of outdoor air pollution in causing asthma is still controversial, it is obvious that asthma attacks increase with increased level of air pollution (45). Indoor pollutants e.g., smoke and fumes from gas and biomass fuels, molds, cockroach infestations are also related with triggered asthma symptoms (1).
Diet
Breast-feeding is the most studied subject in development of asthma. It is shown that infant fed formulas of intact cow?s milk or soy protein have a higher incidence of wheezing illness in early childhood compared with those fed by breast milk (3). Some features of diet such as increased use of processed foods and decreased antioxidant, increased n-6 polyunsaturated fatty acid, and decreased n-3 polyunsaturated fatty acid intakes may be related to increase in asthma (1,46).
DIAGNOSIS of ASTHMA
The most important goal in order to be successful in asthma treatment is establishing a correct diagnosis (1). Clinical history is very important and diagnosis of asthma is prompted by episodic symptoms such as episodic breathlessness, wheezing and chest tightness (47). Daytime and seasonal variability of symptoms, triggering with fog, smoke, smell and exercise, increase at night and response to appropriate asthma treatment support asthma diagnosis (34). A positive family history of asthma and atopic diseases are also helpful diagnostic guides.
If the patient has no symptoms the physical examination of the respiratory system may be normal but asthma diagnosis can not be excluded. The most usual physical finding is wheezing and ronchi on auscultation. Coughing at the end of each inspiration during clinical and physical examination can be an indirect marker of bronchial hyperresponsiveness and may lead clinician to think asthma diagnosis. In severe asthma exacerbations wheezing and ronchi may be absent. However, patients in this state usually have other physical signs reflecting severity, such as cyanosis, drowsiness, difficulty in speaking, tachycardia, hyperinflated chest, use of accessory muscles and intercostal retractions (1).
TESTS for DIAGNOSIS and FOLLOW UP
Asthma can often be diagnosed on the basis of symptoms. However, measurement of lung function supports the diagnosis by assessing the severity of airflow limitation, reversibility and variability in lung function. Lung function test results in normal ranges can not exclude the diagnosis of asthma. Although there was no strong correlation between symptoms and control parameters with lung function tests both in adults and children, these measurements provide descriptive information for asthma control (48,49).
A wide range of different methods to assess the level of airflow limitation exist, but two methods have found widespread acceptance in patients over 5 years age. These are the measurement of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and measurement of peak expiratory flow (PEF). Any FEV1/ FVC value less than 75% are suggestive of airflow limitation (1,48). In a patient who has airflow obstruction a 12% or 200 mL improvement in FEV1 in respect of basal value or a 20% improvement in PEF value after the inhalation of short acting beta2 agonists (4 puff salbutamol= 400 ?g or 4 puff terbutaline = 1000 ?g) indicates the early reversibility of airflow obstruction (1,49,50).
Some of the airflow obstructions have reversibility after 2-3 weeks oral corticosteroid (20-40 mg/day prednisolone) or 6-8 weeks appropriate dose inhaler corticosteroid treatment. If there is a 15% increase in FEV1 value, this indicates the late reversibility. Reversibility can not be found in patients who are under treatment (1).
PEF is still considered as an important aid in the diagnosis and subsequent treatment of asthma (1). Ideally PEF should be first thing that measured in the morning when values are usually close their lowest and last thing at night after taking bronchodilator treatment when values are usually at their highest (1). A diurnal variation in PEF of more than 20% is considered to be diagnostic for asthma (1). Methods to measure PEF variablity are mentioned in the original document (34).
For patients with symptoms consistent with asthma, but with normal lung function, measurements of airway hyperresponsiveness to methacholine, histamine, or exercise challenge may help establishing a diagnosis of asthma (1,50,51,52).
The airway inflammation associated with asthma may be evaluated by examining spontaneously produced or induced sputum for total cell counts, eosinophils, neutrophils and mediators (1,50,53,54). In addition levels of exhaled nitric oxide (NO) or carbon monoxide (CO) have been suggested as non-invasive markers of airway inflammation in asthma (1,50,55).
Evaluation of allergic status in suspected patient from clinical history is skin prick test. The test results should correlate with history in order to carry clinical value. The standard allergens in a prick test examination are; positive/negative control, grass polen, dermatofagoides pteronyssinus, cat and alternaria allergens (38). Measurement of specific IgE is less sensitive and more expensive. Measurement of total IgE in serum has no value as a diagnostic test for atopy.
ASTHMA MEDICATIONS
The effectiveness of drug therapy in asthma has been established for many years. The goal of asthma treatment is to minimize symptoms with the fewest possible adverse effects. The pharmaceutical agents used for asthma can be classified into two main groups; relievers and controllers (1,34).
Available controller medications in Turkey are inhaled glucocorticosteroids, leukotriene receptor antagonists, long-acting β2-agonists, theophylline, anti-IgE and systemic glucocorticosteroids. Available reliever medications are rapid-acting inhaled β2-agonists, systemic glucocorticosteroids, anticholinergics, theophylline, short-acting oral β2-agonists. And the commonly available delivery systems are the metered dose inhaler (MDI), with or without the use of a spacer, dry powder inhalers and nebulizers (56,57).
Clinical effects and side effects for asthma medications are broadly given in Turkish Thoracic Society Asthma Guideline (34).
Controller Medications
Inhaler corticosteroids (ICS) are the most effective controller therapy and must be used continuously (58-64). Higher doses may be required for patients who smoke. Adding a second controller medication is prefered to increasing the dose of ICS (65,66). Common side effects of ICS are mentioned in the original document (34,67,68,69,70,71,72,73,74,75,76,77,78). There is no evidence that use of inhaled glucocorticosteroids increases the risk of pulmonary infections including tuberculosis (79,80). Daily and equivalent doses of ICS available in Turkey are given in Table 4.
Leukotriene Receptor Antagonists (LTRA), are mild bronchodilator and anti-inflammatory drugs, used as an alternative treatment in mild persistent asthma, in some patients with aspirin-sensitive asthma and exercise induced asthma. They can be used as add-on therapy to reduce the dose of ICS required by patients with moderate to severe asthma (81,82,83,84,85,86,87,88,89,90,91). They are effective not only in asthma but also in patients with allergic rhinitis (92). Common side effects are mentioned in the original document (34). Churg-Strauss syndrome associated with LTRA treatment is accepted probably as the result of reductions in the doses of systemic and/or inhaled glucocorticosteroids (93,94,95,96).
Long Acting 2 Agonists (LABA), should not be used as monotherapy in asthma. Clinical control is achieved faster when they are added to ICS (97,98,99,100,101,102). Formoterol + budesonide combination can be used as both reliever and controller therapy (103,104,105,106,107,108). Common side effects are mentioned in the original document (34,109,110,111,112).
Theophylline, it is a mild anti-inflammatory and mild bronchodilator agent. It can be added to ICS if adequate control cannot be achieved with ICS, but less effective than LABA (113,114,115,116,117,118,119,120). Common side effects are mentioned in the original document (34,121).
The principal indication for anti-IgE treatment is uncontrolled severe, allergic asthma cases under high doses of inhaled steroids and other controller therapies, with total IgE level between 30-700 IU (1,122). Anti-IgE has steroid sparing affect and improves asthma control in these selected group of asthmatics (123,124,125,126).
Long-term oral glucocorticosteroid therapy (longer than two weeks) may be required for severely uncontrolled asthma, but its use is limited by the risk of significant adverse effects. The therapeutic index (effect/side effect) of long-term inhaled glucocorticosteroids is always more favorable than long-term systemic glucocorticosteroid therapy in asthma. Oral preparations are preferred over parenteral (intramuscular or intravenous) for long-term therapy because of their lower mineralocorticoid effect, relatively short half-life, and lesser effects on striated muscle, and the greater flexibility of dosing that permits titration to the lowest acceptable dose that maintains control (1,127,128). Common side effects are well known and mentioned in the original document (34,96,129,130,131,132,133).
Allergen specific immunotherapy with clinically relevant allergens may be considered if disease activity is inadequately controlled by avoidance of the allergens and pharmacotherapy (1). Immunotherapy should be avoided when asthma is poorly controlled. Neither should immunotherapy be initiated nor the dosage increased during pregnancy. Common side effects are mentioned in the original document (34).
Reliever Medications
Rapid-acting inhaled β2-agonists are for relief of bronchospasm during acute exacerbations of asthma and for the pretreatment of exercise-induced asthma. They include salbutamol and terbutaline. Because of its rapid onset of action formoterol is also approved for symptom relief, but it should only be used for patients on regular maintenance therapy with inhaled glucocorticosteroids (1).
Systemic glucocorticosteroids are important in the treatment of severe acute exacerbations because they prevent progression of the asthma exacerbation, reduce the need for referral to emergency department and hospitalization, prevent early relapse after emergency treatment, and reduce the morbidity of the illness. Oral therapy is preferred and is as effective as intravenous hydrocortisone (1). The main effects of systemic glucocorticosteroids in acute asthma are evident after 4 to 6 hours. A typical short course of oral glucocorticosteroids for an exacerbation is 30 mg prednisolone given daily for 5 to 10 days depending on the severity of the exacerbation.
Inhaled ipratropium bromide for relief of bronchospasm is less effective than rapid-acting inhaled -agonists in asthma. But it can be used together with an inhaled -agonist as it shows statistically significant improvement in pulmonary function, and significantly reduces the risk of hospital admission (1).
Short-acting theophylline or aminophilline may provide no additive bronchodilator effect over adequate doses of rapid-acting -agonists, but it may benefical for stimulation of respiratory drive and diaphragmatic function (1).
The role of complementary and alternative medicine in adult asthma treatment has not been validated. They are not suggested for routine treatment of asthma in Turkey (1,34).
ASSESSMENT, TREATMENT and MONITORING of ASTHMA
Currently asthma treatment is focused on disease control (1,50,133,134,135). ?Control? adjusted asthma treatment has three domains: assessment of asthma control, treatment to achieve control and monitoring to maintain control (1). In patients taking controller medications, the level of asthma control will guide decisions either to maintain or to adjust therapy (ie, step up if necessary, step down if possible Asthma control levels and control assessment parameters used in the national guideline are shown in Table 5 (1,34,136,137,138,139,140). In treatment naive patient we recommend to begin treatment according to asthma severity (34,50). Mild intermittent asthmatics should receive treatment from step 1 and as severetiy increases initiation step should increase respectively. However the follow-up should be done according to the asthma control (1,34,50).
Treatment steps to achieve asthma control
Step 1: As-needed reliever medication is used for occasional asthma symptoms. We recommend the use of rapid acting inhaled 2-agonist as the first choice (141).
Step 2: We recommend the use of regular controller medication from this step on. The first choice is low dose inhaler glucocorticosterids, alternative controller medication include leukotriene receptor antagonists. Controller medications should be combined with as needed reliever treatment (142,143,144,145).
Step 3: Low dose of inhaled glucocorticosterids combined with a long-acting 2 agonist is the first treatment option (1). Medium dose inhaled glucocorticosterids, low dose inhaled glucocorticosterids with a leukotriene receptor antagonist or sustained release theophylline are alternate regimens (135,146,147,148,149,150). All the regimens should be combined with as needed reliever treatment. If a combination inhaler containing formoterol and budesonide is selected, it may be used for both maintenance and reliever medication (1,151,152,153,154). We emphasize the importance of using long acting β2 agonists always with an inhaled glucocorticosteroids in asthma treatment.
Step 4: Asthmatics who are not controlled on Step 3 should be referred to an experienced centre for asthma management. First treatment option is medium dose of inhaled glucocorticosterids combined with a long-acting 2 agonist (135,146,155,156). In patients whom control can not be achieved with this regimen a third drug like leukotriene receptor antagonist or sustained release theophylline could be added to the treatment (1,157-159). If control is still not achieved, then high dose of inhaled glucocorticosterids combined with a long-acting 2 agonist is another option.
Step 5: This step includes severe and hard to control asthmatics who need further evaluation in an experienced centre for asthma management. Oral glucocorticosterids and anti-IgE can help to achieve control in selected patients (160,161,162,163,164).
Ideally patients must be assessed in four weeks periods till the asthma control is achieved. Thereafter patients must be seen every three months (1). The medications should be reduced until ?the minimum dose that maintains the control? is reached. Stepping down the treatment should be tailored according to the patient?s combination of medications and doses that were needed to achieve control.
We recommend the following suggestions for stepping down the asthma therapy in whom the control is achieved for at least three months (1,34):
In patients using inhaled glucocorticosteroids alone, 50% dose reduction should be introduced at three months intervals (165,166,167). If the control is achieved with low dose inhaled glucocorticosteroids, once daily dosing can be administered (168,169). If the patient is using combination therapy, the stepping down strategy should begin with reducing the dose of inhaled glucocorticosteroids (170). When the minimum dose of glucocorticosteroids is reached than the long acting 2 agonist may be stopped. When the asthma remains controlled for one year with the minimum dose of controller medicine, controller therapy may be stopped. However patients must be closely monitored for the recurrence of symptoms.
Treatment should be stepped up in asthmatics that lose control. If repeated doses of rapid acting β2 agonists do not achieve control, short course of oral glucocorticosteroids or alternately four fold or greater increase in the dose of inhaled glucocorticosteroids (for one to two weeks) can be administered (171,172).
Difficult Asthma
Patients who do not have any factors that makes it difficult to control their asthma and who need two or more controller medications and high doses of inhaled glucocorticosteroids (step 4 therapy) and still can not achieve asthma control are considered as difficult asthmatics (1,173). These patients should be referred to an experienced centre for asthma management.
IDENTIFY and REDUCE EXPOSURE to RISK FACTORS
Pharmacologic intervention to treat asthma is highly effective in controlling symptoms and improving quality of life however measures to prevent the development of asthma or asthma symptoms by avoiding or reducing exposure to risk factors should be implemented when possible. Measures to prevent the development of asthma are named as ?primary prevention?, where as efforts focusing on prevention of asthma symptoms and attacks in patients with established asthma are called ?secondary prevention? (34).
Few measures can be recommended for primary prevention of asthma because the development of the disease is complex and incompletely understood. The role of diet, prevention strategies against inhalant allergens, methods towards reducing exposure to house dust mites, exposure to cats, exposure to tobacco smoke, maternal smoking during pregnancy are widely discussed in the original document (34,174,175,176,177,178,179,180,181,182,183,184,185).
There are theoretical possibilities to avoid the development of asthma in subjects in whom allergic sensitization has already occurred. Whether antihistamines can prevent the development of asthma in children with atopic dermatitis remains an area of investigation (186). Allergen specific immunotherapy has been shown to decrease the risk of asthma development in later life in children with allergic rhinitis (187). However these interventions cannot be recommended for wide adoption in clinical practice at this time.
Asthma symptoms may be caused by many factors including allergens, viral infections, pollutants and drugs. Reducing a patient?s exposure to some of these triggers improves the control of asthma and reduces medication needs. Allergens are important environmental factors that can cause symptoms in the sensitized patient (34). However there is conflicting evidence about whether measures to reduce exposure to indoor allergens are effective at reducing asthma symptoms (34,188,189,190,191,192,193,194,195,196,197,198,199).
Several studies have suggested that outdoor pollutants such as; ozone, nitrogen oxides, acidic aerosols and particulate matters aggravate asthma symptoms. For patients with asthma avoiding physical activity in cold weather and high air pollution are practical recommendations for better control of the disease (200). The most important measure in controlling indoor air pollutants is to avoid passive and active smoking. A multicentered national study demonstrated a significant relation between exposure to tobacco smoke and asthma symptoms (28,201).
Occupational exposures account for a substantial proportion of adult asthma. The early identification of occupational sensitizers and the removal of sensitized patients from any further exposure are important aspects of the management of occupational asthma (202). Routine influenza vaccination of patients with asthma does not appear to protect them from exacerbations. The incidence of viral upper respiratory infections was not different between vaccined and non-vaccined asthmatic patients in a national study. However patients with moderate to severe asthma should be advised to receive an influenza vaccination every year or at least when vaccination of the general population is advised (203,204).
ASTHMA EXACERBATIONS in ADULTS
Asthma exacerbation is characterized by progressive increase in dyspnea, wheesing, chest tightness accompanied by worsening of pulmonary functions. Two main factors are responsible for an asthma exacerbation; inadequate antiinflammatory therapy and being exposed to triggering factors (1,205,206,207,208,209,210).
The severity of exacerbations can be determined according to the patient?s clinical presentation, which includes breathlessness, talking pattern, alertness, respiratory rate, accessory muscle retractions, wheezing, pulsus paradoxus, PEF, PaO2, PaCO2 and SaO2 values. Severity is classified into four categories: mild, moderate, severe and life threatening (1,211,212). We emphasized that severity of asthma should not be underestimated as it can be potentially life threatening. Patients who have risk factors for life threatening asthma exacerbations should be encouraged to admit to a physician without delay and should carefully be monitored in emergency setting. These are asthmatics shown in Table 6 (1,50,207,213,214,215,216,217).
Management of Exacerbations
We recommend home management in mild-moderate exacerbations. However, severe exacerbations should be managed in emergency settings (34).
Home management of exacerbations includes recurrent use of short acting β2 agonists (high doses preferably with a spacer) and systemic glucocorticosteroids (1,34,50,215,217,218,219,220,221).
Patient with a severe exacerbation admitted to emergency department should be evaluated promptly. Oxygen therapy (to achieve SaO2> 90%), rapid acting β2 agonists (nebulized or given with a spacer) at regular and short intervals should be administered (1,34,50,213,215,219,221,222,223). Further bronchodilation can be achieved with combination of ipratropium with salbutamol (224,225,226). Systemic glucocorticosteroids should be administered orally or intravenously as they accelerate the resolution of exacerbation (0.5 mg/kg for 7-10 days) (1,34,50,227,228,229).
We recommend the use of intravenous magnesium sulphate infusion, intravenous theophylline infusion consecutively as further therapies (1,50,219,230,231). Detailed list and dosing of medications used in exacerbations are mentioned in the original document (34).
Intensive care unit therapy and mechanical ventilation: Indications for hospitalization in intensive care and mechanical ventilation are (1):
? Poor response to initial therapy at emergency care or worsening of exacerbation,
? Respiratory insufficiency despite oxygen support (PaO2 < 60 mmHg and/or PaCO2 > 45 mmHg),
? Confusion, cyanosis and severe symptoms,
? Cardiac and respiratory arrest.
Non-invasive mechanical ventilation can be administered in selected cases (1).
Patients can be discharged if their symptoms are under control in the last 24 hours with their prescribed home therapy. Inhaler glucocorticosteroids should not be discontinued during the exacerbation. If the patient was not on an inhaler glucocorticosteroid before exacerbation, it should be prescribed before discharge. Systemic glucocorticosteroids should not be discontinued before 7-10 days. Patients should be referred to an asthma specialist after discharge (1,211,221,232,233,234,235,236).
SPECIAL CONSIDERATIONS
Pregnancy; surgery; rhinitis, sinusitis and nasal polyps; occupational asthma, respiratory infections, gastroesophageal reflux and aspirin-induced asthma need to be considered as special considerations.
Asthma and Pregnancy
The most common respiratory system disorder during pregnancy is asthma (4-7%). Pregnancy effects the natural course of asthma as well as asthma can effect pregnancy and delivery. In approximately one-third of women asthma becomes worse; in one-third asthma becomes less severe; and in the other one-third it remains unchanged during pregnancy (1,237,238). Poorly controlled asthma can have an adverse effect on pregnancy may cause maternal and fetal complications (1,50,51,237,238). Asthma control during pregnancy is very important for both mother and the baby.
Using medications to obtain optimal control of asthma is justified even when their safety in pregnancy has not been proven. For most drugs used to treat asthma there is little evidence to suggest an increased risk to the fetus. Inhaled glucocorticosteroids (ICSs), β2-agonists, leukotriene receptor antagonists, specifically montelukast and appropriately monitored theophylline, are not associated with an increased incidence of fetal abnormalities. ICSs have been shown to prevent exacerbations of asthma in pregnancy. Acute exacerbations should be treated aggressively in order to avoid fetal hypoxia (34).
Delivery will not be different than the non-asthmatics, special consideration should be given to analgesia. Asthmatic mother could breat feed her baby while using her medications (51,238).
Surgery
Asthmatic patients are prone to intraoperative and postoperative respiratory complications due to their airway hyperresponsiveness, limitation, and mucus hypersecretion. These complications may change depending on the severity of asthma at the time of surgery, the type of surgery (thoracic and upper abdominal pose the greatest risks), and type of anesthesia (general anesthesia with endotracheal intubation carries the greatest risk). A careful and detailed evaluation should be undertaken several days prior to surgery and pulmonary function should be measured. If FEV1 value is less than 80 percent of the patient?s personal best, a short course of glucocorticosteroids should be considered. Furthermore, patients who have received systemic glucocorticosteroids within the past 6 months should have systemic coverage during the surgical period (100 mg hydrocortisone every 8 hours intravenously) and rapidly reduced 24 hours following surgery (1,50,51,238).
Rhinitis, Sinusitis and Nasal Polyps
Upper airway diseases can influence lower airway function. Although the mechanisms associated with this relationship are not established, inflammation likely plays a similarly critical role in the pathogenesis of rhinitis, sinusitis, and nasal polyps, as seen in asthma.
Asthma and rhinitis often coexist in the same patient (50,92). The majority (like 75 %) of patients with asthma have a history or evidence of rhinitis and rhinitis frequently precedes the development of asthma (92). Treatment of rhinitis may improve asthma symptoms. Anti-inflammatory agents including glucocorticosteroids, leukotriene modifiers, and anticholinergics can be effective in both conditions (50,92,239).
Both acute and chronic sinusitis can worsen asthma. (92,240,241). Topical nasal decongestants or topical nasal or even systemic glucocorticosteroids should be used to reduce nasal congestion (1,50,92).
Nasal polyps associated with asthma and rhinitis are often accompanied with aspirin sensitivity (92). Between 36% and 96% of aspirin-intolerant patients have polyps and 29% to 70% percent of patients with nasal polyps may have asthma. Nasal polyps respond well to topical corticosteroids, surgery could be considered in non-responders (1,242,243,244).
Occupational Asthma
More than 400 causative agents for occupational asthma have been reported from developed countries (1,238). Recording on occupational diseases has been started in Turkey since 1970 and various occupational exposures have been reported (245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273). Symptoms and air flow limitation while at work and improvement outside the work will lead the diagnosis (34). Spirometric confirmation such as PEF meter follow-up is necessary for legal procedures with a sensitivity of 70-80% and specifity of 85-90%. Gold standard is specific bronchoprovocation but it could only be performed in special centers (50,51,238). Once diagnosed, complete avoidance of the relevant exposure is an important component of management. Continued exposure may lead to severe and potentially fatal asthma exacerbations and permanently impaired lung function. Medical treatment is not different than asthma (50,51,238).
Respiratory Infections
Viral and rarely bacterial infections of respiratory tract may increase symptoms and trigger exacerbations in asthmatics (274,275,276). As increased asthma symptoms often last for weeks beyond the infection, anti-inflammatory treatment should be continued for weeks to ensure adequate control (277,278,279).
Gastroesophageal reflux (GER), is nearly three times as prevalent in all patients with asthma in comparison to the general population (280,281). Most of these patients also have a hiatal hernia; furthermore, theophylline and oral β2-agonists may increase the likelihood of symptoms by relaxing the lower esophageal ring. Patients who are not well controlled with appropriate medical treatment should be evaluated and if necessary treated for GER (282,283,284,285,286,287,288).
Aspirin-Induced Asthma (AIA) is often together with rhinosinusitis, nasal polyp and aspirin intolerance (289). The majority of patients first experience symptoms during the third or fourth decade of life, which may include vasomotor rhinitis and profuse rhinorrhea. Chronic nasal congestion evolves, and physical examination often reveals nasal polyps. Asthma and intolerance to aspirin often develop subsequently. The intolerance itself presents a unique picture: within an hour following ingestion of aspirin, an acute, often severe asthma exacerbation develops, which may be accompanied by rhinorrhea, conjunctival irritation, and scarlet flush of the head and neck. Indeed, a single aspirin or other cyclooxygnease inhibitor can provoke violent bronchospasm, shock, loss of consciousness, and respiratory arrest (1,243,290,291,292,293). Although a patient's clinical history may raise suspicion of AIA, the diagnosis is only established by aspirin challenge, conducted in facilities where cardiopulmonary resuscitation capabilities exist. Patients with AIA should avoid aspirin, products containing it, and other analgesics that inhibit cyclooxygenase and hydrocortisone hemisuccinate. Glucocorticosteroids continue to be the mainstay of therapy, while leukotriene modifiers may be useful for additional control of the underlying disease (294,295,296,297,298,299,300,301,302,303,304,305,306,307,308).
CONFLICT of? INTEREST
None declared.
REFERENCES
- Global Strategy for asthma management and prevention. 2009 (update) www.ginasthma.org
- Braman SS. The global burden of asthma. Chest 2006; 130: 4S-12S. [?zet] [Tam Metin] [PDF]
- Demir AU, Karakaya G, Bozkurt B, Sekerel BE, Kalyoncu AF. Asthma and allergic diseases in schoolchildren: third cross-sectional survey in the same primary school in Ankara, Turkey. Pediatr Allergy Immunol 2004; 15: 531-38. [?zet]
- Demir E, Tanac R, Can D, Gulen F, Yenigun A, Aksakal K. Is there an increase in the prevalence of allergic diseases among schoolchildren from the Aegean region of Turkey. Allergy Asthma Proc 2005; 26: 410-4. [?zet]
- Ones U, Akcay A, Tamay Z, Guler N, Zencir M. Rising trend of asthma prevalence among Turkish school children (ISAAC phases I and III). Allergy 2006; 61: 1448-53. [?zet] [Tam Metin] [PDF]
- Bayram I, Guneser-Kendirli S, Yilmaz M, Altintas DU, Alparslan N, Bingol-Karakoc G. The prevalence of asthma and allergic diseases in children of school age in Adana in Southern Turkey. Turk J Pediatr 2004; 46: 221-5. [?zet]
- Saraclar Y, Kuyucu S, Tuncer A, Sekerel BS, Sackesen C, Kocabas C. Prevalence of asthmatic phenotypes and bonchial hyperresponsiveness in Turkish schoolchildren: an International Study of Asthma and Allergies in Childhooh (ISAAC) phase 2 study. Ann Allergy Asthma Immunol 2003; 91: 477-84. [?zet]
- Von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy 1998; 28(Suppl 5): S45-9. [?zet]
- Sensthilselvan A, lawson J, Rennie DC, Dosman JA. Stabilization of an increasing trend in physician-diagnosed asthma prevalence in Saskatchewan, 1991 to 1998. Chest 2003; 124: 438-48. [?zet] [Tam Metin] [PDF]
- Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics 2002; 110(2Pt 1): 315-22. [?zet]
- Von HertzenL, Haahtela T. Signs of reversing trends in prevalence of asthma. Allergy 2005; 60: 283-92. [?zet] [Tam Metin] [PDF]
- Akcakaya N, Kulak K, Hassanzadeh A, Camcioglu Y, Cokugras H. Prevalence of bronchial asthma and allergic rhinitis in Istanbul school schildren. Eur J Epidemiol 2000; 16: 693-9. [?zet]
- Kendirli GS, Altintas DU, Alparslan N, Akmanlar N, Yurdakul Z, Bolat B. Prevalence of childhood allergic diseases in Adana, Southern Turkey. Eur J Epidemiol 1998; 14: 347-50. [?zet]
- Selcuk ZT, Caglar T, Enunlu T, Topal T. The prevalence of allergic diseases in primary school children in Edirne, Turkey. Clin Exp Allergy 1997; 27: 262-9. [?zet]
- Unlu M, Orman A, Dogan N. The prevalence of asthma among secondary school students in Afyon, Turkey. Asian Pac J Allergy Immunol 2002; 20: 1-6. [?zet]
- Ece A, Ceylan A, Saraclar Y, Saka G, Gurkan F, Haspolat K. Prevalence of asthma and other allergic diseases among school children in Diyarbakir, Turkey. Turk J Pediatr 2001; 43: 286-92. [?zet]
- Alper Z, Sapan N, Ercan I, Canitez Y, Bilgel N. Risk factors for wheezing in primary school children in Bursa, Turkey. Am J Rhinol 2006; 20: 53-63. [?zet]
- Karaman O, Turgut CS, Uzuner N, Olmez D, Babayigit A, Kose S, Tezcan D. The determination of asthma, rhinitis, eczema, and atopy prevalence in 9- to 11-year-old children in the city of Izmir. Allergy Asthma Proc 2006; 27: 319-24. [?zet]
- Zeyerk CD, Zeyrek F, Sevinc E, Demir E. Prevalence of asthma and allergic diseases in Sanliurfa, Turkey, and the relation to environmental and socioeconomic factors: is the hygiene hypothesis enough? J Investig Allergol Clin Immunol 2006; 16: 290-5. [?zet] [PDF]
- Tomac N, Demirel F, Acun C, Ayoglu F. Prevalence and risk factors for childhood asthma in Zonguldak, Turkey. Allergy Asthma Proc 2005; 26: 397-402. [?zet]
- Ozdemir N, Ucgun I, Metintas S, Kolsuz M, Metintas M. The prevalence of asthma and allergy amoung university freshmen in Eskisehir, Turkey. Respir Med 2000; 94: 536-41. [?zet]
- Celik G, Mungan D, Bavbek S, et al. The prevalence of allergic diseases and atopy in Ankara, Turkey: a two-step population based epidemiological study. J Asthma 1999; 36: 281-90. [?zet]
- Tug T, Acik Y. Prevalence of asthma, asthma-like and allergic symptoms in the urban and rural adult population in Eastern Turkey. Asian Pac J Allergy Immunol 2002; 20: 209-11. [?zet]
- Akkurt I, Sumer H, Ozsahin SL, Gonlugur U, Ozdemir L, Dogan O, et al. Prevalence of asthma and related symptoms in Sivas, Central Anatolia. J Asthma 2003; 40: 551-6. [?zet]
- Dinmezel S, Ogus C, Erengin H, Cilli A, Ozbudak O, Ozdemir T. The prevalence of asthma, allergic rhinitis and atopy in Antalya, Turkey. Allergy Asthma Proc 2005; 26: 403-9. [?zet]
- Sakar A, Yorgancioglu A, Dinc G, Yuksel H, Celik P, Dagyildizi L, et al. The prevalence of asthma and allergic symptoms in Manisa, Turkey. Asian Pac J Allergy Immunol 2006; 24: 17-25. [?zet]
- Turktas I, Selcuk ZT, Kalyoncu AF. Prevalence of asthma-associated symptoms in Turkish children. Turk J Pediatr 2001; 43: 1-11. [?zet]
- Kurt E, Metintas S, Basyigit I, Bulut I, Coskun E, Dabak S, et al. Prevalence and risk factors of allergies in Turkey: results of a multicentric cross-sectional study in children. Pediatr Allergy Immunol 2007; 18: 566-74. [?zet]
- Kurt E, Metinta? S, Basyigit I, Bulut I, Coskun E, Dabak S, et al. Prevalence and risk factors of allergies in Turkey (PARFAIT): results of a multicentre cross-sectional study in adults. Eur Respir J 2009; 33: 724-33. [?zet] [Tam Metin] [PDF]
- Celik GE, Bavbek S, Pasaoglu G, Mungan D, Abado?lu O, Harmanci E, et al. Direct medical cost of asthma in Ankara, Turkey. Respiration 2004; 71: 587-93. [?zet]
- Beyhun NE, Cilingiroglu N, Sekerel BE. The cost of childhood asthma and its determinants in Ankara, Turkey. Turk J Pediatr 2007; 49: 179-88. [?zet]
- Beyhun NE, Soyer OU, Kuyucu S, Yildirim S, Boz AB, Altinel N, et al. A multi-center survey of childhood asthma in Turkey - I: the cost and its determinants. Pediatr Allergy Immunol 2009; 20: 72-80. [?zet] [Tam Metin] [PDF]
- Sackesen C, Karaaslan C, Keskin O, Tokol N, Tahan F, Civelek E, et al. The effect of polymorphisms at the CD14 promoter and the TLR4 gene of asthma phenotypes in Turkish children with asthma. Allergy 2005; 60: 1485-92. [?zet] [Tam Metin] [PDF]
- T?rk Toraks Derne?i Ast?m Tan? ve Tedavi rehberi. Turkish Thoracic Journal 2009; 10(Suppl 9): S1-75 .
- Guler N, Kirerli E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol 2004; 114: 254-9. [?zet]
- Sekerel BE, Civelek E, Karabulut E, Yildirim S, Tuncer A, Adalioglu G. Are risk factors of childhood asthma predicting disease persistence in early adulthood different in the developing world? Allergy 2006; 61: 869-77. [?zet] [Tam Metin] [PDF]
- Mungan D, Celik G, Bavbek S, Misirligil Z. Pet allergy: how important for Turkey where there is a low pet ownership rate. Allergy Asthma Proc 2003; 24: 137-42. [?zet]
- Kalyoncu AF, Coplu L, Selcuk ZT, Emri AS, Kolacan B, Kocabas A, et al. Survey of the allergic status of patients with bronchial asthma in Turkey: a multicentric study. Allergy 1995: 5: 451-5. [?zet]
- Akcakaya N, Cokugras H, Camcioglu Y, Ozdemir M. Skin test hypersensitivity for childhood asthma in ?stanbul during a period of 16 years. Allergol Immunopathol 2005; 33: 15-9. [?zet]
- Mungan D, Celik G, Sin B, Bavbek S, Demirel Y, Misirligil Z. Characteristic features of cockroaches sensitivity in Turkish asthmatic patients. Allergy 1998; 53: 870-3. [?zet]
- Uzel A, Capan N, Canbakan S, Yurdakul AS, Dursun B. Evaluation of the relationship between cockroach sensitivity and house-dust sensitivity in Turkish asthmatic patients. Respir Med 2005; 99: 1032-7. [?zet]
- Celik G, Sin B, Keskin S, Ediger D, Bavbek S, Mungan D, et al. Risk factors determining allergic airway diseases in Turkish subjects. J Asthma 2002; 383-90. [?zet]
- Kalyoncu AF, Demir AU, Ozcakar B, Bozkurt B, Artvinli M. Asthma and allergy in Turkish university students: two cross-sectional surveys 5 years apart. Allergol Immunopathol (Madr) 2001; 29: 264-71. [?zet]
- Yildiz F, Ozesen C, Disci R and PASTE study group. Prevalance of asthmatic smokers: Turkish experience (PASTE study). J Asthma (in press).
- Bayram H, Dikensoy O. Air pollution and its effects on pulmonary health. Tuberk Toraks 2006; 54: 80-9. [?zet] [PDF]
- Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol 2005; 115: 1109-17. [?zet]
- Levy ML, Fletcher M, Price DB, Hausen T, Halbert RJ, Yawn BP. International Primary Care Respiratory Group (IPCRG) Guidelines: diagnosis of respiratory diseases in primary care. Prim Care Respir J 2006; 15: 20-34. [?zet] [PDF]
- Kerstjens HA, Brand PL, de Long PM, Koeter GH, Postma DS. Influence of treatment on peak expiratory flow and its relationn to airway hyperresponsiveness and symptoms. The Dutch CNSLD Study Group. Thorax 1994; 49: 1109-15. [?zet] [PDF]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948-68. [?zet] [Tam Metin] [PDF]
- Expert Panel Report 3 (EPR-3): Guidelines fort he diagnosis and management of asthma-Full Report 2007. J Allergy Clin Immunol 2007; 120: 94-138. [?zet]
- The National Asthma Council Australia. Asthma Management Handbook and updated 2006.
- Karaa?a? G, ?elik N, Ba?l?lar S, Y?lmaz T. Diffences in response to direct and indirect stimulus in asthma. Toraks Dergisi 2003; 4: 161-7. [?zet] [Tam Metin] [PDF]
- Karakurt Z, Ceyhan B, Karakurt S, Turker H. Induced sputum cell profile in mild to severe stable asthmatics and healthy adults. Turkish Respiratory Journal 2001; 2: 22-7.
- Yildiz F, Basyigit F, Boyaci H, Ilgazli A, Ozkara S. Comparison of induced cell counts in COPD and asthma. Turkish Respiratory Journal 2003; 4: 43-6.
- O?uz?lgen K, T?rkta? H, Erba? D. Factors effecting the exhaled nitric oxide levels in stable asthmatics. Toraks Dergisi 2002; 3: 232-5. [?zet] [Tam Metin] [PDF]
- Brown PH, Greening AP, Crompton GK. Large volume spacer devices and the influence of high dose beclomethasone dipropionate on hypothalamo-pituitary-adrenal axis function. Thorax 1993; 48: 233-8. [?zet] [PDF]
- Lewis DA, Ganderton D, Meakin BJ, Brambilla G. Modulite: a simple solution to a difficult problem. Respiration 2005; 72 (Suppl 1): 3-5. [?zet]
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroiddependent asthmatics. Am Rev Respir Dis 1990; 142: 832-6. [?zet]
- Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 2000; 343: 1054-63. [?zet] [Tam Metin] [PDF]
- Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen inasthma. A quantitative light and electron microscopic study. Am Rev Respir Dis 1992; 145(4 Pt 1): 890-9. [?zet]
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med 1997; 337: 1405-11. [?zet] [Tam Metin] [PDF]
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343: 332-6. [?zet] [Tam Metin] [PDF]
- Waalkens HJ, Van Essen-Zandvliet EE, Hughes MD, Gerritsen J, Duiverman EJ, Knol K, et al. Cessation of longterm treatment with inhaled corticosteroid (budesonide) in children with asthma results in deterioration. The Dutch CNSLD Study Group. Am Rev Respir Dis 1993; 148: 1252-7. [?zet]
- Jayasiri B, Perera C. Successful withdrawal of inhaled corticosteroids in childhood asthma. Respirology 2005; 10: 385-8. [?zet]
- Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence-based approach. Med J Aust 2003; 178: 223-5. [?zet]
- Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002; 109: 410-8. [?zet]
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med 1999; 159: 941-55. [?zet] [Tam Metin] [PDF]
- Barnes PJ. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol 1998; 102(4 Pt 1): 531-8. [?zet]
- Kamada AK, Szefler SJ, Martin RJ, Boushey HA, Chinchilli VM, Drazen JM, et al. Issues in the use of inhaled glucocorticoids. The Asthma Clinical Research Network. Am J Respir Crit Care Med 1996; 153(6 Pt 1): 1739-48.
- Lee DK, Bates CE, Currie GP, Cowan LM, McFarlane LC, Lipworth BJ. Effects of high-dose inhaled fluticasone propionate on the hypothalamic-pituitary-adrenal axis in asthmatic patients with severely impaired lung function. Ann Allergy Asthma Immunol 2004; 93: 253-8. [?zet]
- Mak VH, Melchor R, Spiro SG. Easy bruising as a side-effect of inhaled corticosteroids. Eur Respir J 1992; 5: 1068-74. [?zet]
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 1902-9. [?zet] [Tam Metin] [PDF]
- Pauwels RA, Yernault JC, Demedts MG, Geusens P. Safety and efficacy of fluticasone and beclomethasone in moderate to severe asthma. Belgian Multicenter Study Group. Am J Respir Crit Care Med 1998; 157(3 Pt 1): 827-32. [?zet] [Tam Metin] [PDF]
- Ernst P, Baltzan M, Deschenes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J 2006; 27: 1168-74. [?zet] [Tam Metin] [PDF]
- Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 1997; 337: 8-14. [?zet] [Tam Metin] [PDF]
- Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or openangle glaucoma. JAMA 1997; 277: 722-7. [?zet]
- Agertoft L, Larsen FE, Pedersen S. Posterior subcapsular cataracts, bruises and hoarseness in children with asthma receiving long-term treatment with inhaled budesonide. Eur Respir J 1998; 12: 130-5. [?zet] [PDF]
- Toogood JH, Markov AE, Baskerville J, Dyson C. Association of ocular cataracts with inhaled and oral steroid therapy during long-term treatment of asthma. J Allergy Clin Immunol 1993; 91: 571-9. [?zet]
- Simons FE, Persaud MP, Gillespie CA, Cheang M, Shuckett EP. Absence of posterior subcapsular cataracts in young patients treated with inhaled glucocorticoids. Lancet 1993; 342: 776-8. [?zet]
- Bahceciler NN, Nuhoglu Y, Nursoy MA, Kodalli N, Barlan IB, Basaran MM. Inhaled corticosteroid therapy is safe in tuberculin-positive asthmatic children. Pediatr Infect Dis J 2000; 19: 215-8. [?zet]
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma 2002; 39: 291-7. [?zet]
- Lipworth BJ. Leukotriene-receptor antagonists. Lancet 1999; 353: 57-62. [?zet]
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999; 340: 197-206.
- Barnes NC, Miller CJ. Effect of leukotriene receptor antagonist therapy on the risk of asthma exacerbations in patients with mild to moderate asthma: an integrated analysis of zafirlukast trials. Thorax 2000; 55: 478-83. [?zet] [Tam Metin] [PDF]
- Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, et al. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Montelukast Asthma Study Group. Eur Respir J 1998; 11: 1232-9. [?zet] [PDF]
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med 1998; 158: 1213-20. [?zet] [Tam Metin] [PDF]
- Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exerciseinduced bronchoconstriction. N Engl J Med 1998; 339: 147-52. [?zet] [Tam Metin] [PDF]
- Dahlen B, Nizankowska E, Szczeklik A, Zetterstrom O, Bochenek G, Kumlin M, et al. Benefits from adding the 5- lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med 1998; 157: 1187-94. [?zet] [Tam Metin] [PDF]
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet JC, Peszek I, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Montelukast/Beclomethasone Additivity Group. Am J Respir Crit Care Med 1999; 160: 1862-8. [?zet] [Tam Metin] [PDF]
- Lofdahl CG, Reiss TF, Leff JA, Israel E, Noonan MJ, Finn AF, et al. Randomised, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ 1999; 319: 87-90. [?zet] [Tam Metin] [PDF]
- Virchow JC, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving highdose inhaled corticosteroids. Am J Respir Crit Care Med 2000; 162(2 Pt 1): 578-85. [?zet] [Tam Metin] [PDF]
- Bousquet J, Khaltaev N, Cruz AA, et al; World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008; 63(Suppl 86): S8-160.
- Wechsler ME, Finn D, Gunawardena D, Westlake R, Barker A, Haranath SP, et al. Churg-Strauss syndrome in patients receiving montelukast as treatment for asthma. Chest 2000; 117: 708-13. [?zet] [Tam Metin] [PDF]
- Wechsler ME, Pauwels R, Drazen JM. Leukotriene modifiers and Churg-Strauss syndrome: adverse effect or response to corticosteroid withdrawal? Drug Saf 1999; 21: 241-51. [?zet]
- Harrold LR, Andrade SE, Go AS, Buist AS, Eisner M, Vollmer WM, et al. Incidence of Churg-Strauss syndrome in asthma drug users: a population-based perspective. J Rheumatol 2005; 32: 1076-80. [?zet]
- Kalyoncu AF, Karakaya G, Sahin A, Artvinli M. Experience of 10 years with Churg-Strauss syndrome: An accompaniment to or a transition from aspirin-induced asthma? Allergol Immunopathol 2001; 29: 185-90. [?zet]
- Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001; 285: 2594-603. [?zet] [Tam Metin] [PDF]
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy vs. continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001; 285: 2583-93. [?zet] [Tam Metin] [PDF]
- Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med 1992; 327: 1420-5. [?zet] [Tam Metin] [PDF]
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. A three-month comparison of twice daily inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. Am Rev Respir Dis 1991; 144 (3 Pt 1): 622-5. [?zet]
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Ann Allergy Asthma Immunol 1998; 80: 463-70. [?zet]
- Stoloff SW, Stempel DA, Meyer J, Stanford RH, Carranza Rosenzweig JR. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol 2004; 113: 245-51. [?zet]
- Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest 2006; 129: 246-56. [?zet] [Tam Metin] [PDF]
- O?Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005; 171: 129-36. [?zet] [Tam Metin] [PDF]
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 2004; 20: 1403-18. [?zet]
- Vogelmeier C, D?Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J 2005; 26: 819-28. [?zet] [Tam Metin] [PDF]
- Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 2007; 61: 725-36. [?zet] [Tam Metin] [PDF]
- Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med 2007; 101: 2437-46. [?zet]
- Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER Jr. Effect of long-term salmeterol treatment on exercise-induced asthma. N Engl J Med 1998; 339: 141-6. [?zet] [Tam Metin] [PDF]
- Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med 1994; 97: 29-37. [?zet]
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129: 15-26. [?zet] [Tam Metin] [PDF]
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004; 364: 1505-12. [?zet]
- Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet 1994; 343: 1006-8. [?zet]
- Kidney J, Dominguez M, Taylor PM, Rose M, Chung KF, Barnes PJ. Immunomodulation by theophylline in asthma. Demonstration by withdrawal of therapy. Am J Respir Crit Care Med 1995; 151: 1907-14. [?zet]
- Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med 2003; 167: 813-8. [Tam Metin] [PDF]
- Rivington RN, Boulet LP, Cote J, Kreisman H, Small DI, Alexander M, et al. Efficacy of Uniphyl, salbutamol, and their combination in asthmatic patients on high-dose inhaled steroids. Am J Respir Crit Care Med 1995; 151(2 Pt 1): 325-32 [?zet]
- Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O'Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high- dose inhaled budesonide for moderate asthma. N Engl J Med 1997; 337: 1412-8. [?zet] [Tam Metin] [PDF]
- Ukena D, Harnest U, Sakalauskas R, Magyar P, Vetter N, Steffen H, et al. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J 1997; 10: 2754-60. [?zet] [PDF]
- Davies B, Brooks G, Devoy M. The efficacy and safety of salmeterol compared to theophylline: meta- analysis of nine controlled studies. Respir Med 1998; 92: 256-63. [?zet]
- Wilson AJ, Gibson PG, Coughlan J. Long acting beta-agonists versus theophylline for maintenance treatment of asthma. Cochrane Database Syst Rev 2000; 2. [?zet]
- Ahn HC, Lee YC. The clearance of theophylline is increased during the initial period of tuberculosis treatment. Int J Tuberc Lung Dis 2003; 7: 587-91. [?zet]
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60: 309-16. [?zet] [Tam Metin] [PDF]
- Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb- E25 Study Group. N Engl J Med 1999; 341: 1966-73. [?zet] [Tam Metin] [PDF]
- Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108: 184-90. [?zet]
- Molimard M, de Blay F, Didier A, Le Gros V. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med 2008; 102: 71-6. [?zet]
- Miller CW, Krishnaswamy N, Johnston C, Krishnaswamy G. Severe asthma and the omalizumab option. Clin Mol Allergy 2008; 20; 6: 4. [?zet] [Tam Metin] [PDF]
- Mash B, Bheekie A, Jones PW. Inhaled vs. oral steroids for adults with chronic asthma. Cochrane Database Syst Rev 2000; 2. [?zet]
- Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Bioequivalent doses of budesonide and prednisone in moderate and severe asthma. J Allergy Clin Immunol 1989; 84(5 Pt 1): 688-700. [?zet]
- Recommendations for the prevention and treatment of glucocorticoid- induced osteoporosis. American College of Rheumatology Task Force on Osteoporosis Guidelines. Arthritis Rheum 1996; 39: 1791-801.
- Campbell IA, Douglas JG, Francis RM, Prescott RJ, Reid DM. Five year study of etidronate and/or calcium as prevention and treatment for osteoporosis and fractures in patients with asthma receiving long term oral and/or inhaled glucocorticoids. Thorax 2004; 59: 761-8. [?zet] [Tam Metin] [PDF]
- Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, et al. A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med 1998; 244: 271-92. [?zet] [Tam Metin] [PDF]
- Guillevin L, Pagnoux C, Mouthon L. Churg-strauss syndrome. Semin Respir Crit Care Med 2004; 25: 535-45. [?zet]
- Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 2004; 114: 40-47. [?zet]
- Sekerel BE, Gemicioglu B, Soriano JB. Asthma insights and reality in Turkey (AIRET) study. Respir Med 2006; 100: 1850-4. [?zet]
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control Study. Am J Respir Crit Care Med 2004; 170: 836-44. [?zet] [Tam Metin] [PDF]
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113: 59-65. [?zet]
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest 1999; 115: 1265-70. [?zet] [Tam Metin] [PDF]
- Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ?well-controlled? and ?not well-controlled? asthma using the Asthma Control Questionnaire. Respir Med 2005.
- Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005; 99: 553-8. [?zet]
- Vollmer WM, Markson LE, O?Connor E, Sanocki LL, Fitterman L, Berger M, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med 1999; 160(5 Pt 1): 1647-52. [?zet] [Tam Metin] [PDF]
- [No authors listed]. Using beta 2-stimulants in asthma. Drug Ther Bull 1997; 35: 1-4.
- O?Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164(8 Pt 1): 1392-7. [?zet] [Tam Metin] [PDF]
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev 2005(1): CD002738. [?zet]
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999; 340: 197-206.
- Deykin A, Wechsler ME, Boushey HA, Chinchilli VM, Kunselman SJ, Craig TJ, et al. Combination therapy with a long-acting -agonist and a leukotriene antagonist in moderate asthma. Am J Respir Crit Care Med 2007; 175: 228-34. [?zet] [Tam Metin] [PDF]
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids, Establishing Therapy (FACET) International Study Group. N Engl J Med 1997; 337: 1405-11. [Tam Metin] [PDF]
- Lofdahl CG, Reiss TF, Leff JA, Israel E, Noonan MJ, Finn AF, et al. Randomised, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ 1999; 319: 87-90. [?zet] [Tam Metin] [PDF]
- Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003; 58: 211-6. [?zet] [Tam Metin] [PDF]
- Fish JE, Israel E, Murray JJ, Emmett A, Boone R, Yancey SW, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2001; 120: 423-30. [?zet] [Tam Metin] [PDF]
- Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O?Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med 1997; 337: 1412-8. [?zet] [Tam Metin] [PDF]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005; 171: 129-36. [?zet] [Tam Metin] [PDF]
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 2004; 20: 1403-18. [?zet]
- Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest 2006; 129: 246-56. [?zet] [Tam Metin] [PDF]
- Vogelmeier C, D?Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J 2005; 26: 819-28. [?zet] [Tam Metin] [PDF]
- Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002; 109: 410-8. [?zet]
- Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence-based approach. Med J Aust 2003; 178: 223-5. [?zet]
- Vaquerizo MJ, Casan P, Castillo J, Perpina M, Sanchis J, Sobradillo V, et al. Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax 2003; 58: 204-10. [?zet] [PDF]
- Virchow JC, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving highdose inhaled corticosteroids. Am J Respir Crit Care Med 2000; 162(2 Pt 1): 578-85. [?zet] [Tam Metin] [PDF]
- Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high-dose inhaled corticosteroid. The Tokyo Joshi-Idai Asthma Research Group. Am J Respir Crit Care Med 1997; 155: 1235-40. [?zet]
- Mash B, Bheekie A, Jones PW. Inhaled vs oral steroids for adults with chronic asthma. Cochrane Database Syst Rev 2000; 2. [?zet]
- Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb- E25 Study Group. N Engl J Med 1999; 341: 1966-73. [?zet] [Tam Metin] [PDF]
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60: 309-16. [?zet] [Tam Metin] [PDF]
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004; 170: 583-93. [?zet] [Tam Metin] [PDF]
- Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A. Differences between asthma exacerbations and poor asthma control. Lancet 1999; 353: 364-9. [?zet]
- Hawkins G, McMahon AD, Twaddle S, Wood SF, Ford I, Thomson NC. Stepping down inhaled corticosteroids in asthma: randomised controlled trial. BMJ 2003; 326: 1115. [?zet] [Tam Metin] [PDF]
- Powell H, Gibson PG. Initial starting dose of inhaled corticosteroids in adults with asthma: a systematic review. Thorax 2004; 59: 1041-5. [?zet] [Tam Metin] [PDF]
- Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev 2004: CD004109. [?zet]
- Boulet LP, Drollmann A, Magyar P, Timar M, Knight A, Engelstatter R, et al. Comparative efficacy of once-daily ciclesonide and budesonide in the treatment of persistent asthma. Respir Med 2006; 100: 785-94. [?zet]
- Masoli M, Weatherall M, Holt S, Beasley R. Budesonide once versus twice-daily administration: meta-analysis. Respirology 2004; 9: 528-34. [?zet]
- Bateman ED, Fairall L, Lombardi DM, English R. Budesonide/formoterol and formoterol provide similar rapid relief in patients with acute asthma showing refractoriness to salbutamol. Respir Res 2006; 7: 13. [?zet] [Tam Metin] [PDF]
- FitzGerald JM, Boulet LP, Follows R, M.A. CONCEPT: a one year, multi centre, randomized double blind, double-dummy comparison of salmeterol/fluticasone propionate using a stable dosing regimen with formoterol/budesonide using an adjustable maintenance regimen in adults with persistent asthma. Clinical Therapeutics 2005; 27: 1-14.
- Reddel HK, Barnes DJ. Pharmacological strategies for selfmanagement of asthma exacerbations. Eur Respir J 2006; 28: 182-99. [?zet] [Tam Metin] [PDF]
- Strek ME. Difficult asthma. Proc Am Thorac Soc 2006; 3: 116-23. [?zet] [Tam Metin] [PDF]
- Friedman NJ, Zeiger RS. The role of breast feeding in the development of allergy and asthma. J Allergy Clin Immunol 2005; 115: 1238-48. [?zet]
- Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr 2001; 139: 261-6. [?zet]
- Mimouni BA, Mimouni D, Mimouni M, Gdalevich M. Does breast-feeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatr 2002; 91: 275-9. [?zet]
- Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life: effect on respiratory symptoms and atopy during the first year of life: a randomised trial. Lancet 2001; 358: 188-93. [?zet]
- Horak F Jr, Matthews S, Ihorst G, Arshad SH, Frischer T, Kueht J, et al. Effect of mite impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth cohort study-24 months results of teh Study of Prevention of Allergy in Children in Europe. Clin Exp Allergy 2004; 34: 1220-5. [?zet]
- van Strien RT, Koopman LP, Kerkhop M, Oldenwening M, de Jongste JC, Gerritsen J, et al. Mattress encasings and mite allergen levels in the Prevention and Incidence of Asthma and Mite Allergy study. Clin Exp Allergy 2003; 33: 490-5. [?zet]
- Marks GB. What should we tell allergic families about pets? J Allergy Clin Immunol 2001; 108: 500-2.
- Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol 2001; 108: 509-15. [?zet]
- Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet 2000; 356: 1392-7. [?zet]
- Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002; 288: 963-72. [?zet] [Tam Metin] [PDF]
- Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development and childhood asthma. Am J Public Health 2004; 94: 136-40. [?zet] [Tam Metin] [PDF]
- Arshad SH. Primary prevention of asthma and allergy. J Allergy Clin Immunol 2005; 116: 3-14. [?zet]
- ETAC Study Group. Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomized, placebo-controlled trial: first results of ETAC. Pediatr Allergy Immunol 1998; 9: 116-24. [?zet]
- M?ller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reducas the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). JACI 2002; 109: 251-6. [?zet]
- Kalpaklioglu F, Emekci M, Ferizli AG, Misirligil Z. On behalf of the House Dust Mite Working Group. A survey of acarofauna in Turkey: comparison of seven different geographic regions . Allergy Asthma Proc 2004; 25: 185-90. [?zet]
- Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen impermeable bed covers for adults with asthma. N Engl J Med 2003; 349: 225-36. [?zet] [Tam Metin] [PDF]
- Halken S, Host A, Niklassen U, Hansen LG, Nielsen F, Pedersen S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. JACI 2003; 111: 169-76. [?zet]
- Gotzsche PC, Hammarquist C, Burr ML. House dust mite control measures in the management of asthma: meta-analysis. BMJ 1998; 317; 1105-10. [?zet] [Tam Metin] [PDF]
- Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev 2004; 4: CD001187. [?zet]
- Bj?rnsdottir US, Jakobinudottir S, Runarsdottir V, Juliusson S. The effect of reducing levels of cat allergen (Fel d 1) on clinical symptoms in patients with cat allergy. Ann Allergy Asthma Immunol 2003; 91: 189-94. [?zet]
- Moira CY, Ferguson A, Dimich-Ward H, Watson W, Manfreda J, Becker A. Effectiveness and compliance to intervention measures in reducing house dust and cat allergen levels. Ann Allergy Asthma Immunol 2002; 88: 52-8. [?zet]
- Carswell F, Oliver J, Weeks J. Do mite avoidance measures affect mite and cat airborne allergens? Clin Exp Allergy 1999; 29: 193-200. [?zet]
- Krieger J, Takaro TK, Allen C, Song L, Weaver M, Chai S, et al. The Seattle-King County Healthy Homes Project: Implementation of a Comprehensive Approach to Improving Indoor Environmental Quality for Low-Income Children with Asthma. Environ Health Perspect 2002; 110(Suppl 2): 311-22. [?zet] [PDF]
- Burr ML, Matthews IP, Arthur RA, Watson HL, Gregory CJ, Dunstan FD, et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax 2007; 62: 767-72 [?zet] [Tam Metin] [PDF]
- Morgan WJ, Crain EF, Gruchalla RS, O?Connor GT, Kattan M, Evans R, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351: 1068-80. [?zet] [Tam Metin] [PDF]
- Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol 2005; 95: 518-24. [?zet]
- Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet 2000; 356: 795-801. [?zet]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ. Respiratory symptom relief related to reduction in cigarette use. J Gen Intern Med 2005; 20: 889-94. [?zet] [Tam Metin] [PDF]
- Rachiotis G, Savani R, Brant A, Mac Neill SJ, Newman-Taylor A, Cullinan P. Outcome of occupational asthma after cessation of exposure: a systematic review Thorax 2007; 62: 147-52. [?zet] [Tam Metin] [PDF]
- Cates CJ, Jefferson TO, Bara AI, Rowe BH. Vaccines for preventing influenza in people with asthma (Cochrane Review). Cochrane Database Syst Rev 2000: CD000364. [?zet]
- Abadoglu O, Mungan D, Pasao?lu G, Cel?k G, Misirligil Z. Influenza vaccination in patients with asthma: effect on the frequency of upper respiratory tract infections and exacerbations. J Asthma 2004; 41: 279-83. [?zet]
- Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest 2004; 125: 1081-102. [?zet] [Tam Metin] [PDF]
- Miles JF, Garden GM, Tunnicliffe WS, Cayton RM, Ayres JG. Psychological morbidity and coping skills in patients with brittle and non-brittle asthma: a case control study. Clin Exp Allergy 1997; 27: 1151-9. [?zet]
- Bavbek S, Celik G, Demirel YS, Misirligil Z. Risk factors associated with hospitalizations for asthma attacks in Turkey. Allergy Asthma Proc 2003; 24: 437-42. [?zet]
- Folkerts G, Buse WW, Nijkamp FP, Sorkness R, Gern JE. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med 1998; 157: 1708-20. [Tam Metin] [PDF]
- Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ 2002; 324: 763. [?zet] [Tam Metin] [PDF]
- Ramnath VR, Clark S, Camargo CA Jr. Multicenter study of clinical features of sudden-onset versus slower-onset asthma exacerbations requiring hospitalization. Respir Care 2007; 52: 1013-20. [?zet] [PDF]
- Mc Fadden ER. Acute severe asthma. Am J Respir Crit Care Med 2003; 168: 740-59.
- Crompton GK. Management of severe asthma. In: Barnes PJ (ed). Asthma, Basic Mechanisms and Clinical Management. 3rd ed. London: Academic Press, 1998: 821-34.
- Janson C, Boe J, Crompton GK. Acute asthma. Eur Respir J 2000; 10: 503-6.
- Romagnoli M, Caramori G, Braccioni F, Ravenna F, Barreiro E, Siafakas NM, et al. Near-fatal asthma phenotype in the ENFUMOSA Cohort. Clin Exp Allergy 2007; 37: 552-7. [?zet]
- Aldington S, Beasley R. Asthma exacerbations: assessment and management of severe asthma in adults in hospital. Thorax 2007; 62: 447-58. [?zet] [Tam Metin] [PDF]
- Bel EH. Management of the acute exacerbation and emergency treatment of asthma. In: Holgate ST, Boushey HA, Fabbri LM (eds). Difficult Asthma. London: Martin Dunitz Ltd., 1999: 227-91.
- Oguzulgen IK, Turktas H, Mullaoglu S, Ozkan S. What can predict the exacerbation severity in asthma? Allergy Asthma Proc 2007; 28: 1-4.
- Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev 2001; CD002988. [?zet]
- Cairns CB. Acute asthma exacerbations: phenotypes and management. Clin Chest Med 2006; 27: 99-108. [?zet]
- Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev 2007; 18: CD000195. [?zet]
- Reddel HK, Barnes DJ. Pharmacological strategies for self-management of asthma exacerbations. Review. Eur Respir J 2006; 28: 182-99. [?zet] [Tam Metin] [PDF]
- FitzGerald JM, Gibsob PG. Asthma exacerbations-4: prevention. Thorax 2006; 61: 992-9. [?zet] [Tam Metin] [PDF]
- Inwald D, Roland M, Kuitert L, McKenzie SA, Petros A. Oxygen treatment for acute severe asthma. BMJ 2001; 323: 98-100. [Tam Metin] [PDF]
- Rowe BH, Edmonds ML, Spooner CH, Camargo CA. Evidence-based treatments for acute asthma. Respir Care 2001; 46: 1380-90. [?zet]
- Rodrigo GJ, Castro J. Anticholinergics in the treatment of children and adults with acute asthma: a Systematic review with meta-analysis. Thorax 2005: 60: 740-6. [?zet] [PDF]
- Rodrigo GJ, Rodrigo C. Triple inhaled drug protocol for the treatment of acute severe asthma. Chest 2003; 123: 1908-15. [?zet] [Tam Metin] [PDF]
- Sherman MS, Verceles AC, Lang D. Systemic steroids for the treatment of acute asthma. Where do we stand? Clin Pulmonary Medicine 2006; 13: 315-20.
- Rodrigo GJ, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma: an evidenced based evaluation. Chest 1999; 116: 285-95. [?zet] [Tam Metin] [PDF]
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients (Review). Cochrane Database Syst Rev 2007.
- Beasley R, Aldington S. Magnesium in the treatment of asthma. Curr Opin Allergy Clin Immunol 2007; 7: 107-10. [?zet]
- Bitz M, Blitz S, Beasley R, Diner BM, Hughes R, Knopp JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev 2005; CD003898. [?zet]
- Bradshaw TA, Matusiewicz SP, Crompton GK, Innes JA, Greening AP. Intravenous magnesium sulfate provides no additive benefit to standart management in acute asthma. Respir Med 2007.
- Rowe BH, Camargo CA. The use of magnesium sulfate in acute asthma: rapid uptake of evidence in North American emergency departments. J Allergy Clin Immunol 2006; 117: 53-8. [?zet]
- Kokturk N, Turktas H, Kara P, Mullaoglu S, Yilmaz F, Karamercan A. A randomized clinical trial of magnesium sulphate as a vehicle for nebulized salbutamol in the treatment of moderate to severe asthma attacks. Pulmonary Pharmacology and Therapeutics 2005; 18: 416-21. [?zet]
- Tapp S, Lasserson TJ, Rowe B. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev. 2007; 18: CD003000. DOI: 1002/14651858. CD003000.pub2. [?zet]
- Foster JM, Hoskins G, Smith B, Lee AJ, Price D, Pinnock H. Practice development plans to improve the primary care management of acute asthma: randomised controlled trial. BMC Fam Pract 2007 24; 8: 23. [?zet] [Tam Metin] [PDF]
- Canadian Asthma Consensus Report, CMAJ 1999; 161: 30: 11.
- British Guideline on Management of Asthma, British Thoracic Society Scottish Intercollegiate Guidelines Network Revised Edition 2005.
- Mungan D. Cooccurrence of asthma and rhinitis. Ankara: Poyraz T?bbi Yay?nc?l?k, 2007; 7: 3.
- Karada? F, ?ilda? O, Pirim C, et al. Frequency of paranasal sinus pathologies and its association with serum eosinophil concentration and IgE levels in asthma. AD? T?p Fak?ltesi Dergisi 2001; 2: 9-12. [?zet] [PDF]
- Nuho?lu Y, ??can M. Nuho?lu ?, et al. Nasal and paranasal sinus tomography findings in asthmatic children with chronic rhitis and recurren sinusites. T?rkiye Klinikleri Allerji-Ast?m Dergisi 2001;3:18-22 [?zet]
- Pata YS, Bicik E, Aygen? E, et al. Late consequences of endoscopic sinus surgery. T?rkiye Klinikleri K.B.B dergisi 2003; 3: 9-15. [?zet]
- Dursun E, Samim E, Korkmaz H, et al. Endoscopic sinus surgery in patients with nasal poliposis. Kulak Burun Bo?az ve Ba? Boyun Cerrahisi Dergisi 1998; 602: 71-80. [?zet] [PDF]
- ?zcan M, Altu? H?, Olcay I et al. Oral corticosteroid use in the treatment of nasal poliposis. Kulak Burun Bo?az ve Ba? Boyun Cerrahisi Dergisi 2000; 8: 83-8. [?zet] [PDF]
- ?zkurt S, Zencir M, Hac?o?lu M, et al. Frequency of occupational asthma auto? painters. Solunum Dergisi 2003; 5: 49-53. [?zet] [PDF]
- U?gun ?, ?zdemir N, Metinta? M, et al. Frequency of occupational asthma in auto? and furniture painters. Solunum Hastal?klar? Dergisi 1999; 10: 126-30.
- Turgut T, Ta?demir C, Muz H, et al. Frequency of occupational asthma in auto? and furniture painters in central Elazig. Tuberk Toraks 2005; 53: 371-8. [?zet] [PDF]
- ??mr?n A, Akp?nar M. Furnisher asthma (two cases). Solunum Hastal?klar? 1997; 8: 99-102.
- Y?lmaz V, K?l??aslan Z, ?lker O, et al. Pulmonary function parameters in auto painters. Solunum 1987; 12: 220-3.
- Fi?ek?i F, K?l??aslan Z. Pulmonary symptoms and prick test findings in furniture painting workers. Solunum Hastal?klar?. 1998; 9: 143-53.
- K?l??aslan Z, Erkan F, Ece T, et al. Baker?s asthma and flour sensitivity in a modern bakery. Solunum 1990; 15: 446-51.
- Top?u F, Yorganc?o?lu A, ??mr?n AH, ?elik P. Occupational asthma prevalence in bakery workers. 25.Y?l Akci?er G?nleri Kongre Kitab? 2000; 332-41.
- Yorganc?o?lu A, ?akar A, Keskin T, Din? G, ?elik P. Respiratory symptoms and occupational asthma in polyurethane foam producers. Turkish Respiratory Journal 2001; 3: 19-23.
- Akp?nar M, ?elikten E, ??mr?n A. Occupational asthma prevalence and risk factors in hairdressers in ?zmir. Solunum Hastal?klar? 1998; 9: 261-8.
- G?lmez ?, ?etinkaya F, Oymak FS, et al. Occupational asthma among hairdresser?s apprentices. Eur Respir J 1998; 12(Suppl 28): S333.
- ?zesmi M, Aslan H. COPD in carpet weavers. Solunum 1984; 9: 260-5.
- K?l??aslan Z, Y?lmaz V, ??kr?k??o?l? S, et al. Pulmonary function impairment in cotton textile workers. Solunum 1987; 12: 242-6.
- G?ven K, ?zesmi M, Demir R. Occupational asthma and wool dust. Solunum 1992; 17: 228-35.
- G?rg?ner M, Mirici A, Girgi? M, et al. Pulmonary symptoms and occupational asthma prevalence in Atat?rk University Carpet Education Centre workers. Solunum 1995; 20: 259-65.
- ?ahin ?, Akkaya A. Pulmonary symptoms and pulmonary function tests in cotton yarn factory workers. Solunum Hastal?klar? 1998; 9: 129-42.
- Er M, Emri S, Karakoca Y, Bar?? Y?. Bissinosis and COPD prevalence in jute yarn factory workers. Toraks Derne?i 2. Kongresi Bildiri ?zet Kitab?. Antalya: 1998; 85.
- Zencir M, Elci OC, Ucku R, Cimrin AH. Prevalence of bysinosis among textile workers. Eur Respir J 1996; 9(Suppl 23): 178.
- Erdo?an S, G?lmez ?,?nl?hizarc? K, et al. Occupational asthma prevalence and pulmonary functions in workers exposed to wood dust. Solunum 1995; 19: 127-34.
- ?uhadaro?lu ?, K?l??aslan Z, Alzafer S, et al. ?stanbul T?p Fak?ltesi ?al??anlar?nda Latex glove allergy in ?stanbul Univesity School of Medicine staff. Solunum 1995; 19: 147-50.
- Ard?? S, ?zdemir N, Cingi M, et al. A new occupational asthma due to morphine powder. Solunum Hastal?klar? 1990; 1: 37-50.
- K?l??aslan Z, Ya?a M. Bronchial asthma associated with detergent enzyme. Eur Respir J 1992; 5(Suppl 15): S405.
- Demirel M, G?lmez ?, Oymak S, Demir R, ?zesmi M. Occupational asthma in glass workers. T?rkiye Solunum Ara?t?rmalar? Derne?i XXV. Ulusal Kongresi, ?stanbul. ?zet Kitab?: SB 044.
- Odaba?? A, Akp?nar M, ?elikten E, El?i ?, Perim K, B?y?k?irin M. Occupational asthma flower sellers. T?rkiye Solunum Ara?t?rmalar? Derne?i XXV. Ulusal Kongresi, ?stanbul. ?zet Kitab?: P 007.
- ?akar A, Kaya E, ?elik P, Gencer N, Temel O, Yaman N, et al. Silikosis in ceramic factory workers. Tuberk Toraks 2005; 53: 148-55. [?zet] [PDF]
- Temel O, ?akar Co?kun A, Yaman N, Sar?o?lu N, Alka? ?, Konyar I, et al. Occupational asthma in welders and painters. World Asthma Meeting 2007 ?stanbul, Abstract ,64.
- Demir AU, Karakaya G, Kalyoncu AF. Allergy symptoms and IgE immune response to rose: an occupational and environmental disease. Allergy 2002; 57: 936-9. [?zet] [Tam Metin] [PDF]
- Tutluoglu B, Ati?, Anakkaya AN, Altug E, Tosun GA, Yaman M. Sensitization to horse hair, symptoms and lung function in grooms. Clin Exp Allergy 2002; 32: 1170-3. [?zet]
- Atis S, Tutluoglu B, Sahin K, Yaman M, K???kusta AR, Oktay I. Sensitization to sunflower pollen and lung functions in sunflower processing workers. Allergy 2002; 57: 35-9. [?zet] [Tam Metin] [PDF]
- Gem JE, Lemanske RF. Infectious triggers of pediatric asthma. Pediatr Clin North Am 2003; 50: 555-75. [?zet]
- Johston SL. Viruses and asthma. Allergy 1998; 53: 922-32.
- Kraft M. The role of bacterial infections in asthma. Clin Chest Med 2000; 21: 301-13. [?zet]
- Weiss ST, Tager IB, Munoz A, Speizer FE. The relation of respiratory infections in early in early childhood to the occurrence of increased levels of bronchial responsiveness and atopy. Am Rev Respir Dis 1985; 131: 573-8. [?zet]
- Buse WW. Respiratory infections: their role in airway responsiveness and pathogenesis of asthma. J Allergy Clin Immunol 1990; 85: 671-83. [?zet]
- Edwards MR, Kabadze T, Johnson MW, Ohston SL. New treatment regimes for virus induced exacerbation of asthma. Pulmonary Pharmacology and Therapeutics 2006; 19: 320-34. [?zet]
- Harding SM. Acid reflux and asthma. Curr Opin Pulm Med 2003; 9: 42-5. [?zet]
- Sontag SJ. Why do the published data fail to clarify the relation between gastroesophageal reflux and asthma? Am J Med 2000; 108: 159-69. [?zet]
- Diette GB, Krishnan JA, Dominici F, Haponik E, Skinner EA, Steinwachs D, et al. Asthma in olders patients. Factors associated with hospitalization. Arch Intern Med 2002; 162: 1123-32. [?zet] [Tam Metin] [PDF]
- Kiljendar TO, Salomoa ER, Hietanen EK, Terho EO. Gastroesophageal reflux in asthmatics. A double blind, placebe controlled crossover study with omeprazole. Chest 1999; 116; 1257-64. [?zet] [Tam Metin] [PDF]
- Irwin RS, Zawacki JK, Curley FJ, French CL, Hoffman PJ. Chronic cough as the sole manifestation of gastroesophageal reflux. Am Rew Respir Dis 1989; 140; 1294-300. [?zet]
- Gibson PG, Henry RL, Coughhlan JL. Gastrooesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev 2003; 2: CD0011496. [?zet]
- Littne MR, Leung WF, Ballard ED, Huang B, Sarma NK. Effect of 24 weeks of lansoprazole theraphy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128: 1128-35. [?zet] [Tam Metin] [PDF]
- Harmanci E, Entok E, Metintas M, Vardareli E, Elbek O. Gastroesophageal reflux in the patiens with asthma. Allergol Immunopathol 2001; 29: 123-8. [?zet]
- Nelson HS. Is gastroesophageal reflux worsening your patients with asthma. Am J Respir Dis 1990; 11: 827-44.
- Samter M, Beers FR. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med 1968; 68: 975-63.
- Szczeklik A, Stevenson DD. Aspirin induced asthma: advance in pathogenesis, diagnosis and management. J Allergy Clin Immunol 2003; 111: 913-21. [?zet]
- Jenkins C, Costello J, Hodge L. Systematic review of prevalence of asprin induced asthma and its implications in clinical practice. BMJ 2004; 328: 434. [?zet] [Tam Metin] [PDF]
- Kalyoncu AF, Karakoca Y, Demir AU, Alpar R, Shehu V, Coplu L, et al. Prevalance of asthma and allergic disease in Turkish university students. Allergol Immunopathol 1996; 24: 152-7. [?zet]
- Celik G, Mungan D, Ozer F, Ediger D, Bavbek S, Sin B, et al. Clinical feature and atopy profile in Turkish subjects with analgesic intolerance. J Asthma 2002; 39: 101-6. [?zet]
- Szczeklik A, Nizankowskka E, Duplaga M. Natural history of asprin induced asthma. AIANE Investigators. European Network on Asprin-Induced Asthma. Eur Respir J 2000: 16: 432-6. [?zet]
- Szczeklik A, Sanak M, Nizankowska-Mogilnicka E, Kielbasa B. Asprin intolerance and the cyclooxygenase-leukotriene patways. Curr Opin Pulm Med 2004; 10: 51-6. [?zet]
- Stevenson DD. Diagnosis, prevention, and treatment of adverse reactions to asprin and nonsteroidal anti-inflammatuary drugs. J Allergy Clin Immunol 1984; 74: 617-22. [?zet]
- Nasser SM, Phister R, Christie PE, Sousa AR, Barker J, Schimitz-Schumann M, et al. Inflammatory cell population in bronchial biopsies from asprine-sensitive asthmatic subjects. Am Respir Crit Care Med 1996; 153: 90-6. [?zet]
- Sampson AP, Cowburn AS, Sladek K, Adamek L, Nizankowska E, Szczeklik A, et al. Profound overexpression of leukotriene C4 synthase in bronchial biopsies from asprine-intolerant asthmatic patients. Int Arch Allergy Immunol 1997; 113: 355-7
- Szczeklik A, Sanak M. Genetic mechanismis in asprine-induced asthma. Am J Respir Crit Care Med 2000; 161: 142-6. [Tam Metin] [PDF]
- Celik G, Bavbek S, Misirligil Z, Melli M. Release of cysteinyl leukotrienes with aspirin stimulation and the effect of prostaglandin E(2) on this release from peripheral blood leucocytes in aspirin-induced asthmatic patients. Clin Exp Allergy 2001; 31: 1615-22. [?zet]
- Dahlen SE, Malstrom K, Nizankowska E, Dahlen B, Kuna P, Kowalski M, et al. Improvement of asprin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double- blind, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 9-15. [?zet] [Tam Metin] [PDF]
- Bavbek S, Celik G, Pasaoglu G, Misirligil Z. Rofecoxib, as a safe alternative for acetyl salicylic acid/nonsteroidal anti-inflammatory drug-intolerant patients. J Invest Allergol Clin Immunol 2006; 16: 57-62. [?zet] [PDF]
- Bavbek S, ?elik G, Ediger D, Mungan D, Demirel YS, Misirligil Z. The use of nimesulide in patients with acetylsalicyclic acid and nonsteroid anti-inflamatory drug intolerance. J Asthma 1999; 36: 657-63. [?zet]
- Bavbek S, Celik G, Ozer F, Mungan D, Misirligil Z. Safety of selective COX-2 inhibitors in aspirin/nonsteroidal anti-inflammatory drug-intolerant patients: comparison of nimesulide, meloxicam, and rofecoxib. J Asthma 2004; 41: 67-75. [?zet]
- Celik G, Pasaoglu G, Bavbek S, Abadoglu O, Dursun B, Mungan D, et al. Tolerability of selective cyclooxygenase inhibitor, celecoxib, in patients with analgesic intolerance. J Asthma 2005; 42: 127-31. [?zet]
- Bavbek S, Dursun AB, Dursun E, Eryilmaz A, Misirligil Z. Safety of meloxicam min aspirin-hypersensitive patients with asthma and/or nasal polyps. A challenge-proven study. Int Arch Allergy Immunol 2006; 142: 64-9.
- [?zet] Celik G, Erkokal FO, Bavbek S, Dursun B, Misirligil Z. Long term use and tolerability of cyclooxygenase- 2 inhibitors in patients with analgesic intolerance. Ann Allergy Asthma Immunol 2005; 95: 33-7. [?zet]
- Drazen JM. Asthma theraphy with agents preventing leukotriene synthesis or action. Proc Assoc Am Physicias 1999; 111: 547-59. [?zet]
Yaz??ma Adresi (Address for Correspondence):
Dr. F?sun YILDIZ,
Kocaeli ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
Umuttepe Merkez Yerle?kesi
41380 Umuttepe, KOCAEL? - TURKEY
e-mail: fusun.yildiz@gmail.com