RESEARCH ARTICLE
Doi: 10.5578/tt.66932
Tuberk Toraks 2018;66(4):317-324
Kronik obstr?ktif akciğer hastalığında pulmoner fonksiyonel parametreler ve kan kotinin d?zeyi
Hatice KOZLUCA1, Emrah DURAL2,3, G?lseren KARABIYIKOĞLU1, T?lin S?YLEMEZOĞLU2
1 Ankara ?niversitesi Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, Ankara, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Ankara University, Ankara, Turkey
2 Ankara ?niversitesi Adli Bilimler Enstit?s?, Adli Toksikoloji Anabilim Dalı, Ankara, T?rkiye
2 Department of Forensic Toxicology, Institute of Forensic Sciences, Ankara University, Ankara, Turkey
3 Sivas Cumhuriyet ?niversitesi, Farmas?tik Toksikoloji Anabilim Dalı, Sivas, T?rkiye
3 Department of Pharmaceutical Toxicology, Faculty of Pharmacy, Sivas Cumhuriyet University, Sivas, Turkey
?ZET
Kronik obstr?ktif akciğer hastalığında pulmoner fonksiyonel parametreler ve kan kotinin d?zeyi
Giriş: Sigara, kronik obstr?ktif akciğer hastalığı (KOAH)'nın en ?nemli nedenidir ve kotinin t?t?n maruziyetinin g?venilir bir g?stergesidir. ?alışmamızda KOAH'lı hastalarda ve kontrol grubu olarak sağlıklı g?n?ll?lerde akciğer fonksiyon parametreleri (%FVC, FEV1, FEV1/FVC ve FEF%25-75), sigara ?yk?s? ve kan kotinin d?zeyi arasındaki ilişkiyi araştırmayı ama?ladık.
Materyal ve Metod: Kliniğimize başvuran 102 KOAH'lı hasta ve 106 sağlıklı g?n?ll? ?alışmaya dahil edildi. Hastaların ve g?n?ll?lerin spirometrik incelemeleri yapıldı. Kan ?rneklerinde kotinin d?zeylerinin belirlenmesi i?in basit, hızlı ve g?venilir bir gaz kromatografi-k?tle spektrometresi (GC-MS) y?ntemi kullanıldı.
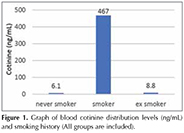
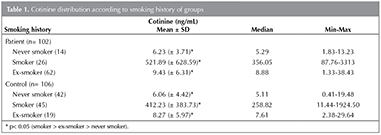
Gere? ve Y?ntem: Kotinin d?zeyi cut-off değeri 41.12 ng/mL olarak belirlendi (%97.2 sensitif ve %100 spesifik). Halen sigara i?en grupta ortalama paket/yıl ve kotinin d?zeyi arasında anlamlı bir ilişki g?zlendi (p< 0.05). Ortalama kotinin d?zeyleri; hi? sigara i?meyen, sigarayı bırakmış ve sigara i?enler i?in sırasıyla 6.1, 8.8 ve 467.0 ng mL-1 idi. Kotinin d?zeyi ile %FVC, %FEV1 ve FEV1/FVC arasında ilişki g?zlenmedi (p> 0.05). Hasta grubunda FEF%25-75 ve kotinin d?zeyi arasında ilişki bulunmamakla beraber sigara i?en kontrol grubunda negatif korelasyon bulundu (p< 0.05; r= -0.372).
Bulgular: ?alışmamızla kotininin t?t?n maruziyetini g?steren g?venilir bir belirte? olduğunu bir kez g?zlemledik. Elde ettiğimiz en belirgin sonu? ise FEF%25-75 değeri ve kotinin d?zeyi arasındaki negatif korelasyondur; bu bulgu KOAH'ın erken evresinde sigaranın periferik hava yollarındaki etkisinden kaynaklanabilir.
Anahtar kelimeler: Kotinin; kronik obstr?ktif akciğer hastalığı (KOAH); sigara; spirometri; gaz kromatografisi k?tle spektrometresi (GC-MS)
SUMMARY
Pulmonary functional parameters and blood cotinine level in chronic obstructive pulmonary disease
Introduction: Smoking is the leading cause of chronic obstructive pulmonary disease (COPD) and cotinine is reliable marker of tobacco exposure. We aimed to investigate the relationship between pulmonary function tests (FVC%, FEV1, FEV1/FVC and FEF25-75%), smoking history and blood cotinine levels in healthy volunteers as a control and patients who have COPD in our study.
Materials and Methods: One hundred and two COPD patients and 106 healthy volunteers who admitted to our institution were included. Spirometric investigations of the patients and volunteers were performed. A simple, rapid and reliable gas chromatography-mass spectrometry (GC-MS) method was used for determination of cotinine levels in blood samples.
Results: The cut-off value of cotinine was determined as 41.12 ng/mL (97.2% sensitivity and 100% specificity). A significant relationship was observed between average pack-year and cotinine level in current smoker group (p< 0.05). The mean cotinine levels were 6.1, 8.8, and 467.0 ng mL-1 in never smokers, ex-smokers and current smokers, respectively. No relationship was observed between cotinine level and FVC%, FEV1% and FEV1/FVC (p> 0.05). In patient group, there was also no relationship between FEF25-75% and cotinine level however, in control group-smokers a negative correlation was found (p< 0.05; r= -0.372).
Conclusion: We observed once again with our study that cotinine is a reliable marker of tobacco exposure. The most obvious result is the negative correlation between FEF25-75% value and cotinine level and this result may be caused by the effect of smoking in the peripheral airways at early stages of COPD.
Key words: Cotinine; chronic obstructive pulmonary diseases (COPD); cigarette; spirometry; gas chromatography-mass spectrometry (GC-MS)
Geliş Tarihi/Received: 11.05.2018 - Kabul Ediliş Tarihi/Accepted: 22.12.2018
INTRODUCTION
Chronic obstuctive pulmonary disease (COPD) is an important cause of chronic morbidity rate and mortality in the world. The most important known risk factor for development of COPD is smoking (1).
Cotinine as the major metabolite of nicotine can be determined in particularly blood, urine and other body fluids including saliva (2).
In our study, we aimed to investigate the relationship between pulmonary function tests (PFTs), smoking history and blood cotinine level in COPD patients and healthy volunteers. For this purpose, a simple and reliable gas chromatography-mass spectrometry (GC-MS) method has been developed to determine in blood cotinine levels in healthy and COPD patients.
MATERIALS and METHODS
COPD patients (n= 102) and healthy volunteers (n= 106) were included in our study. Between 2010 and 2012, volunteers who applied to the our institution were included to our prospective randomized controlled study.
Diagnostic Criteria of Global Initiative for Chronic Obstructive Lung Disease (GOLD) were used for selection of patients in stable period. Demographic characteristics, symptoms and smoking information of the volunteers were recorded. Spirometric examination of the volunteers, which made according to standards, was recorded.
COPD patients were classified into three groups according to smoking history as a current smoker, ex-smoker and never smoker in our study. For determination of the total cigarette consumption, the average pack/year unit was used.
Post-bronchodilator forced vital capacity (FVC%), forced expiratory volume in the first second (FEV1), FEV1/FVC ratio and maximum mid-expiratory flow (FEF25-75% or MMF) values were recorded in the spirometric examination. According to GOLD 2011 criteria, post-bronchodilator FEV1/FVC ratio less than 70% was the main inclusion criteria for patients in our study.
Equipment
The blood cotinine analysis was performed on a 5890-HP gas chromatograph coupled with 5973 MSD detector and equipped with HP-5MS column. Helium was used as carrier gas at 1.0 mL min-1 and 1 ?L sample was applied to column.
Sample Preparation
10 μL of diphenylamine (10 ?g mL-1) as an internal standard and 100 ?L NaOH (1 M) to alkalize the plasma were added to 0.5 mL plasma for calibration and quality control samples. Three mL of ethyl acetate was added to the mixture and samples were agitate in shaking bath for 10 min at 200 rpm and centrifuged at 3000 rpm for 10 min. The upper organic layers were transferred to clean test tubes and they were dried under nitrogen at 40˚C to approximately complete dryness with constant flow. Before the sample loading the GC-MS as 1 ?L volume on splitless mode, it was reconstituted in 350 ?L n-hexane.
Statistical Analysis
Data were analyzed using SPSS version 23 (IBM Corp., Armonk, NY, USA). Evaluation of the data includes both descriptive statistical methods and the Mann-Whitney comparisons, ANOVA, Kruskal-Wallis ANOVA and covariance analysis tests. Nominal variables were assessed by using Pearson's Chi-Square or Fisher's Exact test. Spearman and Pearson correlation test were used for correlation analysis. Receiver Operating Characteristic (ROC) analysis was used for cotinine used as a new marker for separation of smokers and nonsmokers.
Chromatographic Conditions
Gas chromatography (GC) conditions which are optimized were as follows: 1 ?L injection volume using splitless mode was programmed from 70 to 230?C (1 min hold) at rate of 25?C/min. Post run was set at 310?C for 6 min. Total run time was 6.9 min. The interface temperature was set at 280?C. Analytes were quantified at the base peaks of m/z 98, 176 and 352 for cotinine, m/z 169, 168 and 273 for diphenylamine. The retention times of IS and cotinine were 6.05 and 6.45 min, respectively.
Ethical Approval
This study was approved by the Ethics Committee of Ankara University School of Medicine. Written informed consent was obtained from volunteers who participated in the study.
RESULTS
In the study there were 102 people in patient group, which have 85 males (83.3%) and 17 females (16.7%) and 106 volunteers in healthy control group, which have 59 males (55.6%) and 47 females (44.4%). The mean age of the patient group and the control group were 62.1 ? 11.2 (41-87) and 40.9?12.5 (22-78), respectively.
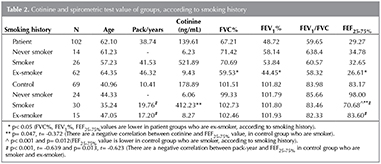
Patient group was consisted of 14 never smokers, 62 ex-smokers and 26 current smokers and control group was consisted of 42 never smokers, 19 ex-smokers and 45 current smokers. Pack/year average was founded 38.7 ? 29.1 and 10.4 ? 12.1 in patient and control group, respectively. Mean daily cigarette consumption of current smokers was 26.5 ? 11.6 in patient group and 19.5 ? 12.5 in control group. Mean pack/year and daily number of cigarettes were observed significantly higher in patient group (p< 0.05). In both groups, mean pack-year were significantly higher in males (p< 0.05). In the patient group a significant correlation was observed between age and pack-year (p< 0.05), despite that similar association was not observed in control group.
One hundred and two patients and 69 healthy volunteers who have spirometric data were analyzed. Mean spirometric values in the patient group and control group were calculated. All spirometric values were significantly lower in patient group (p< 0.001).
Mean plasma cotinine level was observed 139.61 ng/mL (1.33-3313.13 ng/mL) in patient group and 178.9 ng/mL (0.4-1924.5 ng/mL) in control group. No significant difference was observed in cotinine level between two groups. When two groups are considered together, mean cotinine level was 452.39 ng/mL in smokers and 7.91 ng/mL in non-smokers. Cotinine level was determined by ROC analysis, can be used as a reliable indicator to distinguish smokers and non-smokers according to medical history (p< 0.001). Cut-off value was calculated as 41.12 ng/mL. Cotinine level below 41.12 ng/mL indicates "non-smokers" and above 41.12 ng/mL indicate "smokers". Cotinine cut-off value was detected as 100% specific and 97.2% sensitive. Reliability of smoking history was determined 96.0% in patients and 91.5% in control group.
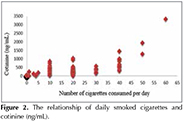
Cotinine level was found that significantly higher in current smokers according to never smokers (p< 0.001) and ex-smokers and also cotinine levels were found highly in ex-smokers according to never smokers (p< 0.05) (Figure 1). Cotinine levels of groups according to smoking history are shown in Table 1. A positive correlation was observed between daily cigarette consumption and cotinine level (p< 0.001; r= 0.741) (Figure 2).
Similar cotinine level was observed between male and female gender. Significant difference in cotinine level was not detected between patient and control groups (p> 0.05). There was no significant relationship between cotinine and age in patient and control groups (p= 0.30).
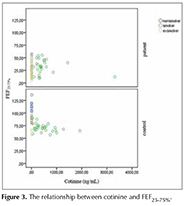
Spirometric results were compared with smoking history and cotinine level. The values of FEV1%, FVC% ve FEF25-75%, were found significantly lower in ex-smokers compared to current smokers and never smokers in patient group (p< 0.05), but a similar relationship was not observed in control group. Unlike this case, the value of FEF25-75% was found that significantly lower in control group who are never smoker and ex-smoker (p< 0.001 and p= 0.012 respectively). While no relationship was detected between pack-year and spirometric result in patient group, a negative correlation was detected between pack-year and FEF25-75% in control group who are current smoker and ex-smoker (p< 0.001; r= -0.639 and p= 0.013; r= -0623, respectively). Consequently, cotinine and spirometric results were compared with adjusted pack-year and smoking history. While, no relationship was found between cotinine and spirometric values in patient group, a negative correlation was detected between the value of cotinine and FEF25-75% in control group who is current smoker (p= 0.047; r= -0.372) (Figure 3). Cotinine values of the both groups according to smoking history and spirometric values are summarized in Table 2.
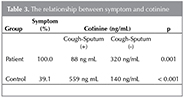
Higher levels of cotinine were correlated with higher incidence of cough and sputum in the control group compared to the patient group (p= 0.001 and p< 0.001) (Table 3).
DISCUSSION
COPD is an important cause of chronic morbidity rate and mortality in the world. Characteristic feature of disease is progressive airflow obstruction. The airflow limitation is associated with an abnormal inflammatory response of the lungs to harmful particles or gases, including tobacco smoke.
Tobacco smoking is the most common addiction in the world and leads to serious illness, which is mainly COPD, as well as many other diseases. Tobacco exposure is closely associated with reduced lung function (1-3).
The main component of tobacco is nicotine and cotinine as the major metabolite of nicotine is considered a good indicator that reflects the active and also passive tobacco exposure (2).
Several analytical techniques, including radioimmunoassay, enzyme-linked immunosorbent assay, gas chromatography-nitrogen phosphorus detection, liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry, high-performance liquid chromatography and gas chromatography-mass spectrometry (GC-MS) for the determination of plasma cotinine are described (4-14).
Chronic shortness of breath, cough and sputum production are the main symptoms of COPD. Due to restriction in airflow, decreased expiratory flow rate is observed in COPD. Spirometry is the first and most widely used in PFTs and also is recognized as the most objective method for determining the airflow limitation (15). PFTs are used to confirm the diagnosis of COPD in patients who have the risk factors and current symptoms.
While the annual FEV1 decline with age in healthy and never smoker is an expected case, this decline in smokers has been increasing about twice as much. The earliest change of airways due to smoking is narrowing of small airways. In this period, FEV1, FEV1/FVC ratio may be in normal range but FEF25-75% reduction, which is reflects small airways restriction, can be observed. Smoking cessation slows the loss of lung function and reduces symptoms (3,16).
Cotinine is the best indicator which is reflecting active or passive tobacco exposure in individuals and cotinine can be detected in many body fluids which are blood, urine and saliva until a week later. In the studies, cotinine level is thought to be an important determinant in predicting the effects of smoking and assessing the risk of airway obstruction (17,18).
When we look at the data which are belonging to Turkey for COPD, according to "Global Adult Tobacco Survey" data which were made with the World Health Organization (WHO) and the Ministry of Health in 2008, 31.3% of the population over age 15 is smoker in Turkey and this ratio was found as 47.9% in men and 15.2% in women (19). According to Turkey-BOLD study in the province of Adana, COPD incidence over age 40 was observed as 19.1%. When these results were analyzed according to sex, incidence was higher more than 3-fold in male gender (20). In our study, majority of volunteers who were ex-smoker and current smoker were male and majority of never smokers (71.4%) were female gender. When we look that the majority of male sex and the smoking rate of the patients, we believe that our study is consistent with the smoking habits and prevalence of male gender in COPD in Turkey.
Studies show that cotinine level is different in various body fluids. While blood and saliva cotinine concentrations indicates positive correlation, urinary cotinine level can be detected about five times higher. However, cotinine concentrations can also vary depending on measurement method (17). In our study, we determined a wide range of plasma cotinine levels (1.3 to 3313.1 ng/mL). Cotinine cut-off value, which is used to determine smoking, shows variety among the studies. Although a value of 10 ng/mL has been widely used as cut-off in previous studies, the values between 10 to 100 ng/mL have been reported. Different cut-off values between the studies are thought to be the effect of cotinine metabolism, race and age (17). The cut-off value was determined as 41.12 ng/mL in our study and this value was100% sensitive and 97.2% specific for determination of exposure.
In studies high and low exposure cut-off values in passive smokers are evaluated in serum as 12 ng/mL and 3 ng/mL respectively. Baltar et al. measured serum cotinine levels in 859 volunteers who were active and passive smokers. The result of a one-hour passive exposure is an increase of 0.45 ng/mL in cotinine level, and the result of one more cigarette in smokers is an increase of 3.95 ng/mL in serum level (21). In our study, there were 137 volunteers who were non-smokers and their cotinine levels were below the 41.12 ng/mL. 19 non-smoker participants' cotinine level were above 12 ng/mL and this situation was considered as a passive exposure.
In many studies, cotinine measurement is used to indicate the reliability of individual smoking anamnesis. Hilberink et al. compared 60 COPD patients' urinary cotinine levels and smoking history (22). Although 12 patients described themselves as non-smoker, high cotinine level was observed in their blood samples and the study showed that anamnesis reliability was 78.0% (22). In our study, anamnesis is found to be reliable which in patient group as 96.0% and control group as 91.5%.
Vineis et al., suggested that, ex-smokers are more sensitive to passive exposure because of they have higher levels of cotinine, according to never smokers (23). Similar results were obtained in our study; cotinine level was significantly greater in ex-smokers than never smokers. It has been thought that ex-smokers have still passive or active exposure or sensitive to passive exposure.
Studies have shown that cotinine level was higher in males and older ages. The presence of lower cotinine level in females depends on the faster nicotine metabolism influenced by sex hormones (24). Cotinine levels were found also different according to the gender in our study.
In the literature, there are very few studies investigating the relationship between PFTs and cotinine. Wu et al. studied the effect of tobacco exposure on lung function. Spirometric values of 402 volunteers were evaluated, FEV1 and FEV1/FVC values in smokers and passive smokers was found significantly lower than non-smokers (25). Vasankar et al. have investigated the relationship between serum cotinine levels and bronchial obstruction in their study. Significant correlations were observed between the number of cigarettes and pack-years and also between age and cotinine. According to spirometry, FEV1% result is less than 80 indicates bronchial obstruction, FEV1% are less than 70 have been accepted as the severe bronchial obstruction. These values are compared with the cotinine level according to smoking history and elevated cotinine level was correlated with the higher risk of bronchial obstruction (18). In our study, a negative correlation was found between pack-year and FEV1 similar to research of Clark et al. (26).
Spirometric values were detected significantly lower as expected in patient group. The ex-smokers had significantly lower FVC%, FEV1 and FEF25-75% values compared to never smokers and current smokers. Similar relationship was not observed in control group, distinctly FEF25-75% were detected significantly lower in current smokers than ex-smokers and never smokers. Although, there were no relationship detected between pack-year and spirometric results in patient group, negative correlation was observed between pack-year and FEF25-75% value.
Cotinine levels and spirometric results were compared in both groups. Although there was no relationship in patient groups, a negative correlation was observed between cotinine levels and FEF25-75% in control groups (p= 0.047; r= -0.372).
Flouris et al. studied, urine and serum cotinine levels, PFTs, and various inflammatory cytokines of 16 never smoker volunteers and measured these parameters initially, on 1st hour and 3rd hour (27). Most of those parameters were found that they returned to basal levels on the 3rd hour however a significant increase in cotinine and some cytokine levels was monitored. This study showed that acute effects of smoking are observed immediately after exposure on lung function tests and then cotinine level reaches the peak value. According to obtained results, it can explain the failure to find any relationship with the other spirometric parameters except for FEF25-75% in our study.
Niewoehner et al. firstly drew attention to inflammation and epithelial changes in respiratory and terminal bronchioles and the term of "peripheral airway disease" was used in first time (28). This situation, was considered as a pioneer of COPD, and if it is detected early mechanisms can be reversible so progression of disease can be prevented. The practical value of the small airway function tests is doubtful. In spirometric screening, while the FEV1 and FEV1/FVC ratio are normal range, FEV3 and FEF25-75% is abnormally low. FEF25-75% is the simplest and most commonly used parameter, in the functional assessment of small airways but significant disadvantages are weak reproducibility and variability depending on expiration volume and expiration time (16). In our study, only relationship between cotinine level and smoking history was a negative correlation between FEF25-75% and cotinine level and pack-year. This result indicates the small airway disease. We suggest that, the decline in FEF25-75% values is associated with peripheral airway obstruction caused by smoking. In the literature, there are no study that examine the relationship between cotinine and FEF25-75%.
Clark et al. observed that patients with productive cough have higher serum cotinine level in their study (26). We also investigated the relationship between the level of cotinine, smoking history and symptoms in patients and control group. While cough, sputum was seen less frequent in patient group, in the control group cough and sputum were found significantly higher with high cotinine level and more pack -years. (p= 0.001 and p< 0.001, respectively). These results in control group could be explained with respiratory symptoms caused by smoking. But, the group of patients with high levels of cotinine had less symptoms. This situation can be explained by the loss of the driving force that allows the extraction of mucus with cough reflex due to loss of cilia that occurs with chronic irritation caused by smoking in the airway in patients.
Our study results also showed that cotinine is a reliable marker to reveal the tobacco exposure Smoking can influence pathophysiology of COPD in many aspects. A negative correlation between the value of FEF25-75% and cotinine level suggests an early effect of smoking on the peripheral airways, in healthy smoker individuals. However, no significant difference in other flow rate parameters especially in COPD patients can be explained with different ways. An inflammatory process unrelated to smoking might be one of these ways. On the other hand, in our study the time elapsed between the last cigarette smoking and taking blood sample for cotinine level, and the time for Spirometric measurements have not been evaluated. As a result, we believe that further designed studies are needed to reveal the real effect of cotinine.
Acknowledgements
Authors would like to thank to Aydın Ciledag, Volkan Kozluca and Ankara University Forensic Toxicology Department Personnel, for the excellent and open collaboration.
REFERENCES
- Global Intitative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [Updated 2013] Available from: http://www.goldcopd.com
- Jung S, Lee IS, Kim SB, Moon CS, Jung JY, Kang YA, et al. Urine Cotinine for Assessing Tobacco Smoke Exposure in Korean: Analysis of the Korea National Health and Nutrition Examination Survey (KNHANES). Tuberc Respir Dis (Seoul) 2012:73:210-8.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977:1:1645-8.
- Knight GJ, Wylie P, Holman MS, Haddow JE. Improved 125I radioimmunoassay for cotinine by selective removal of bridge antibodies. Clin Chem 1985:31:118-21.
- Benkirane S, Nicolas A, Galteau MM, Siest G. Highly sensitive immuno-assays for the determination of cotinine in serum and saliva. Comparison between RIA and an avidin-biotin ELISA. Eur J Clin Chem Clin Biochem 1991:29:405-10.
- Bjercke RJ, Cook G, Rychlik N, Gjika HB, Van Vunakis H, Langone JJ. Stereospecific monoclonal antibodies to nicotine and cotinine and their use in enzyme-linked immunosorbent assays. J Immunol Methods 1986:90:203-13.
- Feyerabend C, Russell MA. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol 1990:42:450-2.
- Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, et al. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers 2010:15:345-52.
- Jacob P, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom 1991:20:247-52.
- Mahoney GN, Al Delaimy W. Measurement of nicotine in hair by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 2001:753:179-87.
- Jacob P 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem 2008:80:8115-21.
- Bernert JT Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997:43:2281-91.
- Hariharan M, VanNoord T. Liquid-chromatographic determination of nicotine and cotinine in urine from passive smokers: comparison with gas chromatography with a nitrogen-specific detector. Clin Chem 1991:37:1276-80.
- Massadeh AM, Gharaibeh AA, Omari KW. A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers' blood and urine samples by RP-HPLC and GC-MS. J Chromatogr Sc 2009:47:170-7.
- Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. Br Med J 2003:327:653-4.
- Milic-Emili J. Does mechanical injury of the peripheral airways play a role in the genesis of COPD in smokers? Rev Mal Respir 2004:1:85-92.
- Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, et al. Assessing secondhand smoke using biological markers. Tobacco Control 2012:22:164-71.
- Vasankari T, Jousilahti P, Knekt P, Marniemi J, Heistaro S, Lppo K, et al. Serum cotinine predicts bronchial obstruction regardless of self-reported smoking history. Scand J Public Health 2011:39:547-52.
- Global Adult Tobacco Survey, Turkey Report, Ministry of Health, Publ. No 803, 2010.
- Kocabas A, Hancioglu A, Turkyilmaz S, Unalan T, Umut S, Cakir B, et al. Prevalence of COPD in Adana, Turkey (BOLD-Turkey Study). Proceedings of the American Thoracic Society 2006; 3 (Abstract Issue): A543.
- Baltar VT, Xun WW, Chuang SC, Relton C, Ueland PM, Vollset SE, et al. Smoking, secondhand smoke, and cotinine levels in a subset of EPIC cohort. Cancer Epidemiol Biomarkers Prev 2011:20:869-75.
- Hilberink SR, Jacobs JE, van Opstal S, van der Weijden T, Keegstra J, Kempers PLj, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med 2011:4:85-90.
- Vineis P, Airoldi L, Veglia F, Olgiati L, Pastorelli R, Autrup H, et al. Environmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the EPIC prospective study. Br Med J 2005:330:1-5.
- Huang CL, Lin HH, Yang YH. Smoking characteristics and saliva cotinine levels in Taiwanese smokers: gender differences. J Clin Nurs 2008:17:2367-74.
- Wu CF, Feng NH, Chong IW, Wu KY, Lee CH, Hwang JJ, et al. Second-hand smoke and chronic bronchitis in Taiwanese women: a health-care based study. BMC Public Health 2010:10:1-10.
- Clark KD, Wardrobe-Wong N, Elliott JJ, Gill PT, Tait NP, Snashall PD. Cigarette smoke inhalation and lung damage in smoking volunteers. Eur Respir J 1998:12:395-9.
- Flouris AD, Metsios GS, Carrillo AE, Jamurtas AZ, Gourgoulianis K, Kiropoulos T, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med. 2009:179:1029-33.
- Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 1974:291:755-8.
Yazışma Adresi (Address for Correspondence)
Dr. Hatice KOZLUCA
Denizli Devlet Hastanesi,
G?ğ?s Hastalıkları Kliniği,
DENİZLİ - T?RKİYE
e-mail: haticetaslak2706@hotmail.com