REVIEW
Doi: 10.5578/tt.35288
Tuberk Toraks 2017;65(2):131-137

Hepatit C ve pulmoner fibrozis
Oğuzhan OKUTAN1, ?mer AYTEN1
1 Haydarpaşa Sultan Abd?lhamid Eğitim ve Araştırma Hastanesi, İstanbul, T?rkiye
1 Clinic of Chest Diseases, Haydarpasa Sultan Abdulhamid Training and Research Hospital, Istanbul, Turkey
?ZET
Hepatit C ve pulmoner fibrozis
Hepatit C halen yery?z?nde en ?nemli infeksiyon ajanlarından biridir. Pulmoner fibrozis ve hepatit C arasındaki ilişkiyi g?stermeye y?nelik yapılan ?alışmalarda ?elişkili sonu?lar elde edilmiştir. Hepatit C'nin pulmoner fibrozis gelişiminde ve alevlenmelerinde rol oynadığı d?ş?n?lse de şu ana kadar yapılan ?alışmalarda bu ilişki net olarak ortaya konulamamıştır. G?n?m?zde hepatit C'nin pulmoner fibrozis gelişimi ?zerine direkt etkilerinden ziyade diğer ekstrahepatik manifestasyanlarına benzer indirekt etkilerinden s?z edilmektedir. Ancak bu indirekt etkiler bile sadece olgu bazlı ?alışmalarda g?sterilebilmiştir.
Anahtar kelimeler: Hepatit C, pulmoner fibrozis, interferon alfa
SUMMARY
Hepatitis C and pulmonary fibrosis
Hepatitis C is one of the most important infectious agents worldwide. There are conflicting results regarding the relationship between pulmonary fibrosis and hepatitis C. It is thought that hepatitis C may play a role in the development or exacerbations of idiopathic pulmonary fibrosis, but no clear link between hepatitis C and pulmonary fibrosis development has been demonstrated yet to date. In the recent era, indirect effects of hepatitis C rather than a direct effect are more suspected on pulmonary fibrosis. These indirect effects could also been documented only by a few case-based studies.
Key words: Hepatitis C, pulmonary fibrosis, interferon alpha
Geliş Tarihi/Received: 11.11.2016 - Kabul Ediliş Tarihi/Accepted: 28.11.2016
INTRODUCTION
The lack elucidation of the pathogenesis of Idiopathic pulmonary fibrosis (IPF) causes several etiological agents to be accused. The recent assumed hypothesis for IPF is the alveolar epithelial cell damage and resulting abnormal epithelial-mesenchymal response, which is developed after recurrent exposure to unknown environmental antigenic substances in susceptible individuals. Several etiological viral agents such as Herpes simplex virus type 1 (HSV-1), Epstein-Barr virus (EBV), Cytomegalovirus (CMV) are investigated, however, one of the most investigated virus is hepatitis C virus (1,2).
Hepatitis C is one of the most important infectious agents, due to its current high prevalence, mortality and morbidity rates. More than 170 million people worldwide are infected with hepatitis C virus, which corresponds to approximately 3% of the whole world population. Chronic hepatitis develops in 50-80% of cases after the acute infection of the virus, and these patients are faced with many hepatic (cirrhosis, portal hypertension, hepatocellular carcinoma, liver failure) and extrahepatic manifestations. IPF is one of the reported more than 30 extrahepatic complications associated with virus (Table 1). Hepatitis C is a lymphotropic virus and it is thought that with chronic immune system activation it can cause fibrotic effects in the lungs like in the liver (3-5).
The Relation of Hepatic and Pulmonary Fibrosis in Hepatitis C Infection
Hepatic fibrosis is a common manifestation in chronic hepatitis C infection and the level of fibrosis is very important for treatment and prognosis. Although some non-invasive tests have been launched in the recent years, liver biopsy is still the gold standard. The most common used scoring system for liver fibrosis evaluation is METAVIR scoring system: F0: no fibrosis, F1: portal fibrosis without septa, F2: few septa, F3: numerous septa without cirrhosis F4: cirrhosis. Fibrosis scores ≥ F2 are thought to have severe liver fibrosis. Pretreatment high fibrosis level (Metavir F3-F4) is important for assuming treatment outcomes (6). The prevalence of these levels are not clearly documented. In their meta-analysis, Theinet al. reported fibrosis rates as F0: 17%, F1: 35%, F2: 22%, F3: 14% and F4: 12%, and they also demonstrated that 15-30% of patients with chronic hepatitis C will come up against face up with cirrhosis in 10-30 years (7). In 5232 patients with chronic hepatitis C who underwent liver biopsy, Arase et al. reported fibrosis rates as 44%, 19%, 4.8% and 16% for F1, F2, F3 and F4, respectively. Among all patients 15 (%0.3) developed IPF in 10 years follow-up period (8).
Fibrotic effects of hepatitis C virus (HCV) in liver start up with hepatocellular damage. Direct cytopathic effects of the virus, and TH1 mediated intrahepatic persistent immune response and related cytokines are responsible for the hepatocellular damage related with HCV infection. Increased TH1 response in HCV infection is not sufficient for virus elimination, and unfortunately is responsible for a nonspecific chronic inflammation leading chronic liver damage. Resulting increased immune system activation ends with hepatocellular damage and increased hepatocyte turnover. Finally, the imbalance between increased hepatocellular apoptosis and regeneration results with hepatic fibrosis and cirrhosis. Several proinflammatory, proapoptotic and proproliferative gene expressions and some individual factors also play a role for cirrhosis development (9). The development of cirrhosis is quicker in patients who receive > 50 g alcohol daily, who have concomitant hepatitis B and human immunodeficiency virus (HIV) infection, who are male and who infected elderly (7,10). Interestingly, cirrhosis risk is decreased in patients who infected with HCV in early decades. With other words, there is no link between the long duration of HCV infection and the increased risk for hepatic fibrosis and cirrhosis development. The variability of juvenile and elder host immune responses are thought to be responsible for this condition (11,12).
Another important factor in hepatic fibrosis and cirrhosis development is the hepatocyte specific telomere shortening. In chronic hepatitis C patients, hepatocyte death and regeneration lead acceleration in hepatocyte specific telomere shortening. This condition results in decreased regeneration capacity in hepatocytes and progression of fibrosis (13).
Pulmonary fibrosis in IPF is thought to be secondary to a viral or an unknown origin antigenic entity causing repeated alveolar epithelial and tissue damage, tissue regeneration, inflammation and fibrosis. Previously it is thought that fibrosis in IPF was similar with the fibrotic process seen in liver, but more recently low response rates to anti-inflammatory treatment modalities moved us away from this hypothesis. In the recent era, the chronic inflammation in IPF is thought to be secondary to fibrosis (14).
While a TH1 mediated immune response is responsible in hepatic fibrosis secondary to chronic HCV infection, TH2 mediated immune response and associated increased cytokines like IL-4, IL-5 ve IL-13 are in the foreground in IPF (15).
In normal circumstances, damage in alveolar epithelial cells results in replacement of type 2 alveolar epithelial cells with damaged type 1 alveolar epithelial cells. Type 2 alveolar cells increase and cover the damaged basal membrane, and then differentiate to type 1 alveolar epithelial cells in order to restore the damaged area. In IPF, myofibroblasts accumulate to the damaged area and results in collagen and extracellular matrix deposits. In the course of these events, damaged alveolar epithelial cells cannot be repaired properly and as a response to this condition several growth factors and cytokines are secreted. Excessive secretion of growth factors and fibroblast/myofibroblast induction and regeneration cause a chronic process (16,17).
"Premature aging" is the other important accentuated topic for the pathogenesis of IPF. Telomere length anomalies (telomere shortening) that are commonly seen in familial IPF and the late onset of the disease consists of the main basis oh this hypothesis. It is thought that telomere shortening has a major impact on IPF pathogenesis by leading epithelial and progenitor cell loss and dysfunction (18).
Hepatopulmonary syndrome (HPS) is another pulmonary pathology, which is developed secondary to chronic liver disease. HPS is a syndrome, which can be seen in 15-20% of patients with cirrhosis and characterized with the presence of liver disease, increased alveolar-arterial oxygen gradient and intrapulmonary vasodilatation (19). It is thought that pulmonary vasodilatation is developed secondary to increased vasodilator production like nitric oxide. This process leads impaired ventilation and perfusion and resulting hypoxia (20). In the majority (46-100%) of patients who have HPS, reticulonodular densities can be seen in chest X-Ray (21). In computed tomography, dilated vessels and increased terminal branching extending to the pleura are helpful for differential diagnosis with several parenchymal diseases like IPF. To date there is no known pathogenetic relationship between HPS and IPF, however, there is also no conducted study regarding this topic.
Hepatitis C and Pulmonary Fibrosis
There are conflicting results regarding the relationship between IPF and hepatitis C. Ueda et al. from Japan, were determined a high proportion of HCV antibodies in the serum specimens of the patients with IPF (19 of 66 patients, 28.8%) (22). In 12 of 19 HCV antibody detected patients, HCV infection was confirmed with specific tests. Similarly, Meliconi et al. have determined HCV antibodies in 8 of 60 (13.3%) patients with IPF and reported that this difference was statistically significant when compared with control group (0.3%) (23). HCV-RNA was positive in all patients in whom HCV antibodies were detected. Conversely, Irving et al. detected HCV antibodies in only one of 62 serum samples of patients with IPF (24). In this patient HCV-RNA was also negative. During an average follow-up of 8 years, Yasuji, et al. found that, only 15 of more than 6.000 HCV-infected patients have developed IPF (8). They also found that the risk of new cases for IPF was 0.3% at the end of 10 years and 0.9% at the end of 20 years. The conflicting results between these studies have been linked to the differences between the IPF prevalence among several countries and the false positivity of the tests used to document anti-HCV antibodies. In real, all is confirming the low link between HCV infection and the development of IPF.
Conflicting results were also found in the cellular examination of the bronchoalveolar lavage (BAL) of hepatitis C patients. Idil et al. compared BAL examination of 18 chronic hepatitis C patients with healthy control group (25). They found that total cell count and neutrophil counts were higher but lymphocytes, macrophages and eosinophil counts were similar. They concluded that increased neutrophil count might be due to an unknown inflammatory reaction. In their study on 13 hepatitis C patients, Kubo et al. found that BAL lymphocytes and eosinophil counts were higher when compared with healthy control group, but not total cell counts were similar (26). In another study of the same researchers, the authors investigated the pre-treatment and post-treatment BAL cell counts of the hepatitis C patients receiving interferon alpha treatment and compared with healthy individuals. They found that eosinophil and lymphocyte counts were higher in the pre and post treatment groups when compared with control group (27). In the follow-up of 300 chronic HCV patients, Ferri et al. documented that only 8 patients with pulmonary symptoms had decreased carbonmonoxide diffusing capacity (DLCO) and pulmonary interstitial involvement in high-resolution computed tomography (HRCT) (28). They that halve of these patients had increased neutrophil counts in BAL examinations. Okutan et al. documented probable interstitial pulmonary disease signs in HRCT in about half of patients with chronic HCV (16 of 34 patients) infection (29). In the same study, they also documented the decreased DLCO among the majority of study group (26 of 34 patients) when compared with the control group.
Pathogenesis
Although several mechanisms have been postulated for IPF pathogenesis, a clear hypothesis still not put forward. Recently the most suspected mechanisms are alveolar epithelial damage triggered by an unknown exogenous or endogenous agent, impaired tissue healing and fibrosis secondary to recurrent chronic inflammation. In the last few years, it is thought that some unknown infectious agents especially viral agents may play a role in the development or exacerbation of IPF in genetically predisposed individuals. Given the temporal heterogeneity in IPF, active and latent periods in the virus life cycle may have been associated with this heterogeneity. Although gamma herpes viruses, cytomegalovirus, and some other viruses such as hepatitis C is considered to be one of these infectious agents, none has been proven. Despite the animal studies demonstrating the triggering effects of gamma herpes viruses for the development of pulmonary fibrosis, there are no animal studies conducted to investigate the effects of hepatitis C virus (30). But, hepatitis C virus is a lymphotropic virus and may cause a chronic activation on the immune system.This warning was also defined for interstitial lung diseases along with several rheumatic diseases such as rheumatoid arthritis and mixed crioglobulinemia (31). However, no clear link between hepatitis C and pulmonary fibrosis development has been demonstrated. Idilmanet al. suggested that increased BAL neutrophil counts in hepatitis C patients had triggered the the development of alveolitis and the resulting alveolar fibrotic process (25).
Another mechanism is the immunglobulin-immune complex deposition in tissue and systemic circulation or the direct tissue damage secondary to HCV-RNA.In HCV patients, decreased elimination of systemic antibodies and antigens due to liver dysfunction lead accumulation of immune complexes in several tissues. HCV and HCV antibody containing immune complex accumulation in glomeruli are accused for glomerulonephritis developed in patients with HCV. In several studies HCV-RNA positivity was documented in kidney tissue and urine, however, accumulation of HCV in glomeruli could be documented in only one study (32,33).On the contrary, HCV-RNA was detected in only one patient's BAL fluid but not documented in the lung tissue (25,26). The immune complexes in the circulatory system are important in the pathogenesis of IPF, such taht these complexes form a basis for fibroblast proliferation and neutrophil chemotaxis by activating macrophages. Circulating immune complexes are held responsible in the development of mixed crioglobulinemia in HCV patients, and it is thought that IPF is secondarily developed as a complication of mixed crioglobulinemia in some patients with hepatitis C (28).
Pulmonary Fibrosis and Treatment of Hepatitis C Interferon Alpha and Interstitial Pneumonia
Interferons are one of the members of glycoprotein family, which is called cytokines. They are natural proteins produced from immune system cells as a result of antigenic stimulus. They have antiviral (by inhibiting viral replication), immunomodulator (by increasing natural killer cell activity, and by antibody production and B cell differentiation over T helper cell) and anti-proliferative properties. Interferon alpha, interferon beta and interferon gamma are the main three forms used in clinical practice. Interferon alpha, the most common used interferon, already used effectively in the treatment of hepatitis C infection.
Interferon treatment is also widely used in several cancers (malign melanoma, chronic myelocytic leukemia, renal cell carcinoma, carcinoid tumor, multiple myeloma etc.), Behcet's disease, crioglobulinemia and condyloma accuminata. Several systemic (fever, myalgia, vomiting etc.) hematologic (thrombocytopenia, neutropenia etc.) adverse effects, autoimmune and pulmonary complications have been reported due to interferon treatment (34). (Table 2) Ronnblomet al. reported several autoimmune diseases (thyroid dysfunction in 18 patients, pernicious anemia in 4 patients, vasculitis in 2 patients and systemic lupus erythematous in 1 patient) in 25 (19%) of 135 malign carcinoid patients receiving interferon alpha treatment (35). Despite several autoimmune diseases are reported in the patients receiving interferon alpha, interstitial pneumonia is reported only in patients with hepatitis C infection. But debate still goes on whether this interstitial pneumonia was secondary to interferon treatment or due to the autoimmune response directly triggered by hepatitis C virus.
Although the incidence of interferon alpha (IFN) induced interstitial pneumonia is not exactly known, it is estimated as 0.3% in Japan and 0.01% in other countries (36). The mechanism of interferon (IFN) induced pulmonary fibrosishas not been clearly understood, but pathogenesis mainly focuses on the immune stimulatory effects of interferon alpha. It is thought that immune system stimulation secondary to interferon alpha causes accumulation of multiple proinflammatory and profibrotic cytokines, and finally predisposing pulmonary fibrosis (37).
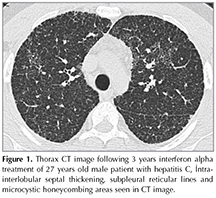
IP may develop at any stage of the interferon treatment and in the 2-48 weeks period after the treatment initiation. The most common symptoms are dyspnea, dry cough, fever, fatigue, arthralgia, and myalgia. Hemoptysis, and wheezing is seen less frequently. After cessation of IFN treatment many of the symptoms resolve in 2 months period (38,39). There are difficulties in the diagnosis of IFN related IP due to lack of definitive diagnostic criteria. In physical examination inspiratory crackles can be auscultated. Patchy infiltrates on chest radiograph and bilateral patchy consolidation are as with ground glass opacities on chest tomography can be observed especially in the acute phase, intra- interlobular septal thickening, subpleural reticular lines and honeycombing areas in chronic phase (38) (Figure 1).
There is no determined standard treatment modality for IFN induced IP. Discontinuation of IFN therapy is the main treatment choice for clinically evident IP. Systemic steroids are the most common agents used for treatment, but patient selection, treatment duration and treatment doses are not clearly documented. However, in the symptomatic cases and in the evidence of progression steroid treatment must be initiated immediately. In some cases, which do not response to steroids and have frequent relapses, the treatment can be combined with azathioprine (38,40). Slavenburg et al. investigated 25 IFN induced IP cases and they demonstrated good response rates to steroid treatments in the majority of cases (38). In this studyreported mortality rate was 7%. IFN induced IP usually demonstrates good response rates to steroid treatment, but high mortality rates must also kept in mind.
New Generation Oral Antiviral Agents and Interstitial Pneumonia
There are no comprehensive studies regarding pulmonary effects of new antiviral agents like NS3/NS4A protease inhibitors (telaprevir, boceprevir, simeprevir), NS5B nucleoside inhibitors (sofosbuvir), NS5B non-nucleoside inhibitors and NS5A inhibitors (ledipasvir, daclatasvir). In a study conducted on 123 patients receiving simeprevir + sofosbuvir + ribavirin treatment for liver transplantation, Pungpapong et al. reported that one patient receiving sofosbuvir + ribavirin treatment was died due to diffuse alveolar damage (41). In this study five patients (4%) complained of dyspnea but all did not required further evaluation. There are only two case reports in the literature except this study. One of them is a patient with IP secondary to simeprevir +? peginterferon and ribavirin combination treatment. The other is a case of diffuse alveolar hemorrhage receiving simeprevir + sofosbuvir treatment for liver transplantation. Steroid treatment was not effective in both cases with diffuse alveolar damage, but the patient with IP responded to 20 mg/day prednisolone treatment (42,43).
CONCLUSION
The role of viruses in the pathogenesis of IPF is still one of the research topics, but the role of hepatitis C virus is outdated and has a falling importance. The lack of new studies conducted in the last decade to evaluate the role of hepatitis C infection in IPF is also an indicator of this situation. The development mechanism of IP in hepatitis C patients is also not elucidated yet. In the recent era, indirect effects of hepatitis C rather than a direct effect are more suspected on pulmonary fibrosis. These indirect effects could also been documented only by a few case-based studies. IFN induced IP is one of the major side effect that can affect the course of treatment and mortality of hepatitis C. Even it is documented by several case studies that steroids are resulted in good treatment responses, it must be kept in mind that they have considerable mortality rates.
REFERENCES
- Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis Eur Respir Rev 2013;22:376-81.
- Okutan O, Ayten O. Tanım ve sınıflama. İdiopatik pulmoner fibrozis. In Kartaloglu Z, Okutan O (Ed). Pulmonary diseases and disorders. 3rd ed. Philadelphia: WB Saunders Company, 1998;1430-2.
- Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Curr Pharm Des 2008;14:1646-54.
- Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection: some current issues. J Hepatol 2004;40: 341-52.
- Zignego AL, Ferri C, Monti M,? LaCivita L, Giannini C, Careccia G, et al. Hepatitis C virus as a lymphotropic agent: evidences and pathogenetic implications. Clin Exp Rheumatol 1995;13(Suppl):S33-7.
- Bedossa P, Bioulac-Sage P, Callard P, Chevallier M, Degott C, et al; The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20(1 Pt 1):15.
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-31.
- Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, et al Hepatitis C virus enhances incidence of idiopathic pulmonary fibrosis.? World J Gastroenterol 2008;14:5880-6.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825-32.
- Karidis NP, Delladetsima I, Theocharis S. Hepatocyte turnover in chronic HCV-induced liver injury and cirrhosis. Gastroenterol Res Pract 2015;2015:654105.
- Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect 2001;3:851-7.
- Gao B, Hong V, Radeva S. Host factors and failure of interferon treatment in hepatitis C virus. Hepatology 2004;39:880-90.
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J,? et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J 2002;16:935-42.
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 2002;3:3.
- Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. In: Fishman AF (ed). Fishman's pulmonary disease and disorders. 4th ed. NY: The McGraw-Hill, 2008;1144-60.
- King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011:378:1949-61.
- Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014;9:157-79.
- Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: Distinct horns of the same devil? Respiratory Res 2012;11;13:3.
- Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med 1995;122:521-9.
- Krowka MJ, Cortese DA. Hepatopulmonary syndrome: current concepts in diagnostic and therapeutic considerations. Chest 1994;105:1528-37.
- McAdams HP, Erasmus J, Crockett R, Mitchell J, Godwin JD, McDermott VG. The hepatopulmonary syndrome: radiologic findings in 10 patients. AJR Am J Roentgenol 1996;166:1379-85.
- Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, et al. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis 1992;146:266-8.
- Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, et al. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax 1996;51:315-7.
- Irving WL, Day S, Johnston ID. Idiopathic pulmonary fibrosis and hepatitis C virus infection. Am Rev Respir Dis 1993;148:1683-4.
- Idilman R, Cetinkaya H, Savaş I, Aslan N, Sak SD, Baştemir M, et al. Bronchoalveolar lavage fluid analysis in individuals with chronic hepatitis C. J Med Virol 2002;66:34-9.
- Kubo K, Yamaguchi S, Fujimoto K, Hanaoka M, Hayasaka M, Honda T, et al. Bronchoalveolar lavage fluid findings in patients with chronic hepatitis C virus infection. Thorax 1996;51:312-4.
- Yamaguchi S, Kubo K, Fujimoto K, Honda T, Sekiguchi M, Sodeyama T. Analysis of bronchoalveolar lavage fluid in patients with chronic hepatitis C before and after treatment with interferon alpha. Thorax 1997;52:33-7.
- Ferri C, La Civita L, Fazzi P, Solfanelli S, Lombardini F, Begliomini E, et al. Interstitial lung fibrosis and rheumatic disorders in patients with hepatitis C virus infection.? Br J Rheumatol 1997;36:360-5.
- Okutan O, Kartaloğlu Z, Ilvan A, Kutlu A, Bozkanat E, Silit E. Values of high-resolution computed tomography and pulmonary function tests in managements of patients with chronic hepatitis C virus infection. World J Gastroenterol 2004;10:381-4.
- Williams KJ. Gamma herpesviruses and pulmonary fibrosis: evidence from humans, horses, and rodents. Vet Pathol 2014;51:372-84.
- Bombardieri S, Paoletti P, Ferri C, Di Munno O, Fornal E, Giuntini C. Lung involvement in essential mixed cryoglobulinemia.? Am J Med 1979:66;748-56.
- Doutrelapont JM, Adler M, Willems M, Durez P, Yap SH. Hepatitis C infection and membranoproliferative glomerulonephritis. Lancet 1993;341;17.
- Yamabe H, Inuma H, Osawa H, Kaizuka M, Tamura N, Tsunoda S, et al. Glomerular deposition of hepatitis C virus in membranoproliferative glomerulonephritis. Nephron 1996;72:741.
- Tanrı?ver MD, S?zen T. İnterferon-tedavisi ve otoimm?nite. Hacettepe Tıp Dergisi 2007;38:39-44.
- R?nnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 1991;115:178-83.
- Solsky J, Liu J, Peng M, Schaerer M, Tietz A. Rate of interstitial pneumonitis among hepatitis virus C-infected patients treated with pegylated interferon. J Hepatol 2009;50(Suppl 1):S238.
- Midturi J, Sierra, Hoffman M, Hurley D, Winn R, Beissner R, et al. Spectrum of pulmonary toxicity associated with the use of interferon therapy for hepatitis C: case report and review of the literature. Clin Infect Dis 2004:39:1724-9.
- Slavenburg S, Heijdra YF, Drenth JP. Pneumonitis as aconsequence of (peg) interferon-ribavirin combination therapy for hepatitis C: a review of the literature. Dig Dis Sci 2010;55:579-85.
- Fan-PuJi, Zheng-Xiao Li, Hong Deng, Hong-An Xue, Yuan Liu, Min Li. Diagnosis and management of interstitial pneumonitis associated with interferon therapy for chronic hepatitis C. World J Gastroenterol 2010;16:4394-9.
- Abi-Nassif S, Mark EJ, Fogel RB, Hallisey RK Jr. Pegylated interferon and ribavirin-induced interstitial pneumonitis with ARDS. Chest 2003;124:406-10.
- Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology 2015;61:1880-6.
- Tamaki K, Okubo A. Simeprevir with peginterferon and ribavirin induced interstitial pneumonitis: First case report. World J Gastroenterol 2015;21:1009-13.
- Helmers RA, Byrne TJ, Wesselius LJ, Leslie KO. Serious pulmonary toxicity secondary to novel hepatitis C antiviral therapy in a liver transplant recipient. Mayo Clinic Proceedings 2015;90:1294-7.
Yazışma Adresi (Address for Correspondence)
Dr. Oğuzhan OKUTAN
Haydarpaşa Sultan Abd?lhamid Eğitim ve Araştırma Hastanesi,
G?ğ?s Hastalıkları Kliniği,
İstanbul, T?rkiye
e-mail: oguzhanokutan@gmail.com