LETTER TO THE EDITOR
Doi: 10.5578/tt.9884
Tuberk Toraks 2016;64(2):182-183

?lkemizden quantiferon Tb gold in tube indeterminant sonu?lari
Yeşim G?ROL1, G?lden ?ELİK1
1 Yeditepe ?niversitesi Tıp Fak?ltesi, Tıbbi Mikrobiyoloji Anabilim Dalı, İstanbul, T?rkiye
1 Department of Medical Microbiology, Faculty of Medicine, Yeditepe University, Istanbul, Turkey
Interferon gamma release assays (IGRAs) are used worldwide alternative to tuberculin skin test (TST). The sensitivity and specificity of these tests are 78-90%, 93-99%. There are some limitations of IGRAs such as indeterminate results. As we use Quantiferon TB Gold in Tube (QFT-2G) in our laboratory, we have also found some indeterminate results especially in some age groups. The aim of our study? was to share the rate of indeterminate results from our country comparing to other studies worldwide.
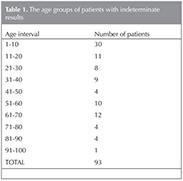
We evaluated the results of? QFT-2G tests during six year period (2006-2012) and we detected 1625 negative, 559 positive and 93 indeterminate test results among 2277 patients. The test was performed as the manufacturer's instructions. The rate of indeterminate result was 4%. The distribution of the indeterminate results according to the age groups were as follows; 30 in 1-10 ages (30.2%), 12 in 61-70 ages (13.04%) (Table 1).
There are few studies about the relationship? between interferon gamma production? and age. In a study of a large? pediatric population, indeterminate results were found more frequent with QFT-IT? than the other IGRA (T SPOT TB) especially in? children < 4 years of age as 12.6% vs 2.3% (1). Although the indeterminate results were reported as follows 1.4%,17% (2,3).
Mori? shared? the significant decrease in QFT positive rate in older age in a review article (4). Kobashi et al. detected the frequency of indeterminate results as 17% (5).
Some reasons are thought to effect the results. Ferrara et al. noted that indeterminate QFT-2G test results appeared in 21% of tests performed in routine clinical microbiological laboratories due to positive control failure and that indeterminate results were significantly overrepresented among patients receiving immunosuppressive treatments. However, there was no speculation concerning the reason (6). Banach found that age, race/ethnicity and sex were associated with high nil and low mitogen of indeterminate results (2%). Although technical factors relating to specimen handling and test performance may influence the indeterminate result rate, their data also showed that biological factors may also play a role (7).
Although it has been indicated that younger children (< 5 yrs old) also tend to show indeterminate QFT-2G test results, this group presented with a normal range of lymphocyte counts and serum protein (8).
There are studies mostly comparing IGRAs with tuberculin skin test in our country. As far as we know there is no data about indeterminate results? published. We have a limitation in this study by only performing QFT-G in tube tests not also with T SPOT TB. So we couldnt compare these two IGRAs to show the correlation? of age and the indeterminate results. The other limitation is this study is a retrospective one. To determine the scale there should be studies of large number patients.
REFERENCES
- Bergamini BM, Losi M, Vaienti F, D'Amico R, Meccugni B, Meacci M, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 2009;123:419-24.
- Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of? a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax 2006;61:616-20.
- Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the Quantiferon- TB Gold In Tube test. Pediatrics 2009;123:30-7.
- Mori T. Usefulness of interferon-gamma release? assays for diagnosing TB infection and problems with these assays. J Infect Chemother 2009;15:143-55.
- Kobashi Y, Mouri K, Yagi S, Obase Y, Miyashita N, Okimoto N, et al. Clinical utility of the Quantiferon TB-2G? test for elderly patients with active tuberculosis. Chest 2008;133: 1196-202.
- Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-c-assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005;172:631-5.
- Banach DB, Harris TG. Indeterminate QuantiFERON?-TB Gold results in apublic health clinic setting. Int J Tuberc Lung Dis 2011;15:1623-9.
- Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J 2009;33:812-5.
Yazışma Adresi (Address for Correspondence)
Dr. Yeşim G?ROL
Yeditepe ?niversitesi Tıp Fak?ltesi,
Tıbbi Mikrobiyoloji Anabilim Dalı,
İSTANBUL - T?RKİYE
e-mail: yesimg@yeditepe.edu.tr