RESEARCH ARTICLE
Doi: 10.5578/tt.10778
Tuberk Toraks 2016;64(2):112-118

Orta evre kronik obstr?ktif akciğer hastalığında uzun etkili antikolinerjik ile inhale steroid-uzun etkili
beta agonistlerin etkinlik ve g?venliliklerinin karşılaştırılması
Pınar SARA?1, Abdullah SAYINER1
1 Ege ?niversitesi Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, Izmir, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Ege University, Izmir, Turkey
* European Respiratory Society International Congress in Munich, Germany, 6-10 September 2014. S?zl? Sunum, T?rk Toraks Derneği 16. Yıllı Kongresi, Belek, Antalya, 3-7 Nisan 2013.
?ZET
Orta evre kronik obstr?ktif akciğer hastalığında uzun etkili antikolinerjik ile inhale steroid-uzun etkili beta agonistlerin etkinlik ve g?venliliklerinin karşılaştırılması
Giriş: Kronik obstr?ktif akciğer hastalığı (KOAH) tedavisi; hastaların sağlık d?zeyini arttırmayı, mortalite, morbidite ve alevlenme gelişimini azaltmayı hedeflemektedir. Bu ?alışmada orta evre KOAH'ı olan hastalarda uzun etkili antikolinerjik ile? inhale steroid/uzun etkili beta-2 agonist kombinasyonunun etkinlik ve g?venliliğinin karşılaştırılması hedeflenmiştir.
Materyal ve Metod: Bu ?alışma FEV1 (Zorlu ekspiratuvar vol?m 1. saniye) değeri %50-80 arasında olan KOAH'lı hastaları i?eren a?ık u?lu, prospektif, randomize bir ?alışmadır. Bigilendirilmiş onamı alınan, alınma ve dışlanma kriterlerini karşılayan 44 hasta ?alışmaya dahil edildi. İki haftalık wash-out periyodu sonunda hastalar bir grup salmeterol 50 ?g/flutikazon propionat 500 ?g kuru toz inhalasyon kombinasyon (discus) tedavisini g?nde iki kez (SF grup) olacak şekilde, diğer grup tiotropium kuru toz inhalasyon (handihaler) tedavisini 18 ?g g?nde bir kez (T grup) olacak şekilde bir yıl s?resince kullanmak ?zere iki gruba randomize edildi. Olgular iki grup arasında eşit olarak dağıtıldı (her bir ?alışma grubunda 22 hasta). İzlem periyodunda hastaların 3, 6, 9 ve 12. aylarda poliklinik kontrolleri yapılması planlandı.
Bulgular: Demografik veriler ve temel ?l??mler a?ısından iki grup arasında istatistiksel olarak anlamlı fark saptanmadı. SF grubunda toplam alevlenme 1.2 ? 1.7, T grubunda toplam alevlenme 2.1 ? 2.2 olarak değerlendirildi (p= 0.070). İlk alevlenmeye kadar ge?en s?re sırasıyla 4.2 ? 4.0 ve? 4.2 ? 3.3 ay olarak değerlendirildi (p= 0.245). Acil servise başvuru ya da hastaneye yatışı gerektiren ciddi alevlenmelerin sayısı sırasıyla 6 ? 1.0 ve 1.1 ? 1.4, olarak değerlendirildi (p= 0.245). Tedavi s?recinde ?nemli gelişmeler her iki grupta da CAT (CPOD Assessment Test) skorlarında g?zlendi (p< 0.0001) , ancak iki grup arasında istatistiksel anlamlı fark saptanmadı.
Sonu?: Bu ?alışma ile orta seviyeli hava yolu obstr?ksiyonu olan hastalarda tedavide inhale kortikosteroid ve uzun etkili beta-2 agonist kombinasyonu kullanımı ile? uzun etkili antikolinerjik kullanımı karşılaştırıldığında pulmoner fonksiyon testlerinde, poliklinik başvurularında ve egzersiz kapasitesinde benzer klinik gelişmeler sağlandığı g?r?lm?şt?r.
Anahtar kelimeler: Kronik obstr?ktif akciğer hastalığı, antikolinerjikler, adrenerjik beta-2 agonistler, kortikosteroidler
SUMMARY
Compare the efficacy and safety of long-acting anticholinergic and a combination of inhaled steroids and long-acting beta-2 agonist in moderate chronic obstructive pulmonary disease
Introduction: The treatment of COPD (Chronic Obstructive Pulmonary Disease) aims to improve the patients's well-being and to reduce mortality, morbidity and the development of exacerbations. This study was thus designed to compare the efficacy and tolerability of salmeterol/fluticasone combination with tiotropium in patients with moderate COPD.
Materials and Methods: This was an open, prospective, randomized trial in COPD patients whose FEV1 (forced expiratory volume in 1 second) levels were between 80% and 50% predicted. A total of 44 patients who met the inclusion and exlusion criteria and who gave written informed consent were included in the study. At the end of the two week wash-out period, the patients were randomized to receive either salmeterol 50 ?g/fluticasone 500 ?g combination as dry powder inhaler twice daily (SF Group) or tiotropium dry powder inhaler 18 ?g once daily (T Group) for one year. These were equally distributed in the two groups (22 patients in each study group). At follow-up, the patients were required to come to the outpatient clinic at the third, sixth, ninth and twelfth months.
Results: There were no statistically significant difference between the two groups with regards to demographic features and baseline measurements. There were 1.2 ? 1.7 exacerbations in SF Group and 2.1 ? 2.2 exacerbations in T Group (p= 0.070). The time to the first exacerbation was 4.2 ? 4.0 and 4.2 ? 3.3 months, respectively (p= 0.697). The number of severe exacerbations that resulted in admission to the emergency department or hospital was 0.6 ? 1.0 and 1.1 ? 1.4, respectively (p= 0.245). Significant improvements were observed in CAT (CPOD Assessment Test) scores in both groups during the treatment period (p< 0.0001); but there was no difference between the two groups.
Conclusion: This study has shown that in patients with moderate COPD, treatment with combined corticosteroid and long-acting beta-2 agonist provides similar improvements in pulmonary function tests, patient-reported outcomes and exercise capacity as compared a long-acting anticholinergics.
Key words: Chronic obstructive pulmonary disease, anticholinergics, adrenergic beta-2 agonists, corticosteroids
INTRODUCTION
The treatment of COPD aims to improve the patients's well-being and to reduce mortality, morbidity and the development of exacerbations (1). Thus, the choice of treatment is based on the severity of symptoms and the risk of developing adverse events.
Although the level of airflow obstruction, as assessed with FEV1 levels, used to be the main parameter to guide treatment, studies have shown that there is a relatively weak correlation between FEV1 levels and measurements of health-related quality of life (2,3). Besides, the strongest predictor of future exacerbations has been found to be the frequency of exacerbations in the preceding year (4). Current guidelines thus recommend the use of multiple parameters, including the frequency of exacerbations, severity of symptoms as well as the degree of airflow obstruction in treatment decisions (5). For patients with moderate airflow obstruction, a long-acting bronchodilator is recommended with or without an inhaled steroid depending on the presence or absence, respectively, of frequent exacerbations.
Beta2-agonists and anticholinergics are the mainstay of bronchodilator treatment. Several recent large trials have improved our understanding of the disease and enabled the clinicians to base their treatment approach on scientific evidence. It has been recently shown that in patients with moderate-to-very severe COPD, tiotropium is more effective than salmeterol in preventing exacerbations (6). However, the TORCH trial previously showed that, in patients with FEV1 levels less than 60%, combination of a long-acting beta2-agonist (LABA) with an inhaled corticosteroid (ICS) leads to more pronounced improvements in lung function, health-related quality of life and rate of exacerbations compared with the use of a beta2-agonist alone (7). In the largest trial comparing tiotropium with salmeterol-fluticasone combination in patients with severe airway obstruction, no difference was found in exacerbation rate, while SGRQ score was higher, the rates of withdrawal and mortality were lower in patients who received ICS/LABA combination (8). To our knowledge, there is no study performed in COPD patients with moderate obstruction that compared the effectiveness of these two frequently used drugs.
This study was thus designed to compare the efficacy and tolerability of salmeterol/fluticasone combination with tiotropium in patients with moderate COPD.
MATERIALS and METHODS?
This was an open, prospective, randomized trial in COPD patients whose FEV1 levels were between 80% and 50% predicted. Consecutive patients were randomized to receive either tiotropium or salmeterol-fluticasone combination for one year. The randomizaton was done according to a list prepared prior to the initiation of the study.
This study was reviewed and approved by the Ethics Committee of Ege University. All participants gave written informed consent.
Study Population
All patients who were seen in the outpatient clinic between May 2010 and June 2011 were evaluated for eligibility for entry into the trial. They were included if they were between 35-80 years old, they had a smoking history of 10 pack-years or more, their FEV1 level was between 50% and 80% and they reported at least one exacerbation in the preceding year. The exclusion criteria were the presence of a prior diagnosis of asthma, previous documentation of bronchial hyperreactivity, history of allergy and/or atopy, presence of congestive heart failure or any other cardiopulmonary disease that might interfere with the patient's follow-up.
Study Protocol
After obtaining the patients' informed consent, the patients entered a two-week wash-out period, when all long-acting bronchodilators and inhaled steroids were stopped and they were only allowed to take short-acting bronchodilators (salbutamol-ipratropium combination MDI). In the second visit at the end of the wash-out period, the demographic features and the baseline measurements were recorded and the patients were randomized to receive either salmeterol 50 ?g/fluticasone 500 ?g combination as dry powder inhaler (discus) twice daily (SF Group) or tiotropium dry powder inhaler (handihaler) 18 ?g once daily (T Group) for one year. The study medications were kindly provided by Glaxo Smith Kline and Boehringer Ingelheim companies. These companies had no other involvement in the planning, design and execution of the study and in the analysis of the data.
The patients were also informed to use short-acting bronchodilators when needed, but were not allowed to use any other bronchodilators or inhaled steroids. In the event of an exacerbation, they could be treated with antibiotics and/or systemic steroids, as recommended by their attending physician. These were then recorded by the study investigators.
Measurements
At baseline, all relevant demographic data including risk factors for COPD, frequency of exacerbations during the preceding year, previous hospitalizations, medications and immunizations were recorded. Besides, post-bronchodilator pulmonary function tests, arterial blood gas measurements, six-minute walk test and COPD Assessment Test (CAT) were performed and the symptom scores were recorded.
At follow-up, the patients were required to come to the outpatient clinic at the third, sixth, ninth and twelfth months. At each visit, all tests and measurements done at baseline were repeated and the patients were questioned about whether they had any exacerbation during the preceding three months. An exacerbation was defined as any worsening of respiratory symptoms for three or more days that necessitated increased use of bronchodilators or additional administration of antibiotics and/or systemic steroids or that resulted in visit to the emergency department and/or admission to the hospital.
Statistical Analysis
Chi-square and Fisher's Exact test were performed for categorical variables. Student's t-test was done for variables which showed a normal distribution, and Mann-Whitney U test was done for variables that did not.
RESULTS
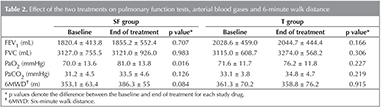
A total of 44 patients who met the inclusion and exlusion criteria and who gave written informed consent were included in the study. These were equally distributed in the two groups (22 patients in each study group). There were no statistically significant difference between the two groups with regards to demographic features and baseline measurements (Table 1). All patients completed the study.
Exacerbations During the Follow-up Period
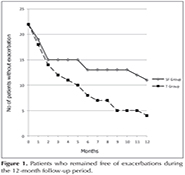
There were 1.2 ? 1.7 exacerbations in SF Group and 2.1 ? 2.2 exacerbations in T Group (p= 0.070). The time to the first exacerbation was 4.2 ? 4.0 and 4.2 ? 3.3 months, respectively (p= 0.697). The number of severe exacerbations that resulted in admission to the emergency department or hospital was 0.6 ? 1.0 and 1.1 ? 1.4, respectively (p= 0.245). There was no difference in patients who remained free of exacerbations during the 12-month follow-up period (Figure 1). There was no difference in exacerbations requiring the use of antibiotics (10 and 11 in SF and T groups, respectively) and of systemic steroids (one exacerbation in each group). There was also no difference in the rate of exacerbations between patients who reported frequent (two or more) and non-frequent exacerbations in the preceding year (data not shown). One patient in SF group developed pneumonia, was hospitalized and recovered following appropriate antibiotic therapy.
Spirometry and Arterial Blood Gas Analysis
No significant changes were observed in pulmonary function tests in either group (Table 2). With regards to arterial blood gas analysis, PaO2 values improved in the SF group only, whereas no significant improvement occurred in the T group (Table 2).
Patient-Reported Outcomes
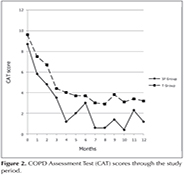
Significant improvements were observed in CAT scores in both groups during the treatment period (p< 0.0001); but there was no difference between the two groups (Figure 2).
No significant changes were seen in mean Borg Dyspnea Scores in SF Group (from 0.8 ? 1.5 to 0.3 ? 0.6, p= 0.262) and in T Group (from 1.0 ? 1.8 to 0.5 ? 1.0, p= 0.169).
Six-Minute Walk Distance
No significant difference was noted in the six-minute walk distances in either group (Table 2).
Adverse Events
No significant adverse event was observed in either group. None of the patients developed pneumonia.
DISCUSSION
This study has shown that in patients with moderate airway obstruction, treatment with an ICS/LABA combination provides similar improvements in pulmonary function tests, patient-reported outcomes and exercise capacity as compared to the use of a long-acting anticholinergic agent. There tended to be fewer exacerbations with SF, but the difference did not reach clinical significance. Similarly, although there was a tendency for improvement in the six-minute walk distance in the SF group, this did not reach statistical and clinical significance. Significant improvements in PaO2 levels were observed in the SF Group only.
The treatment recommendations of the current GOLD guidelines are based on the severity of the symptoms and the potential risk for adverse events, including exacerbations and hospitalizations. Thus, bronchodilators are mainly used to relieve symptoms and to improve the quality of life, whereas inhaled steroids are recommended for patients with severe airflow obstruction and/or frequent exacerbations, who are considered to be at high risk. However, recent reports indicated that COPD patients with moderate disease may obtain important benefits from the combined use of inhaled steroids and long-acting beta agonists (9). Besides, two meta-analyses showed that combination of long-acting beta-agonists with inhaled steroids provide better outcomes as compared to the use of long-acting beta-agonists only (10,11). This is also reflected in prescription rates in real life; i.e. combinations are more frequently prescribed than long-acting beta agonists alone (12,13,14).
Our findings are similar to those of the INSPIRE Study which compared the relative efficacy of treatments with tiotropium and salmeterol/fluticasone combination in patients with severe COPD, in that they were associated with similar decreases in the rate of exacerbations (8). The POET Study, on the other hand, in which nearly half of the study population had moderate airway obstruction, showed that tiotropium was superior to salmeterol in preventing exacerbations (6). The current study thus complements these data, showing that the combination of ICS/LABA provides similar clinical outcomes in patients with moderate obstruction, as compared to the use of tiotropium. This difference in findings is possibly due to the use of an inhaled steroid in this study.
The combination of the inhaled steroid with salmeterol may be associated with additional benefits through its antiinflammatory effects (15,16) and/or the synergistic interaction between the steroids and beta-agonists at the cellular level (17). It is now established that COPD is an inflammatory disease and signs of airway inflammation are observed from the early stages (18). Besides, it has also been shown that inhaled steroids and their combination with long-acting beta-agonists improve clinical outcomes in patients with severe as well as moderate disease (9).
The main weakness of this study is the limited number of patients, which may have caused a type II error. Particularly in view of the trend for fewer exacerbations in the SF group, observations from larger number of patients would surely enable the clinicians to draw firmer conclusions related to patient management. However, the finding that treatment with ICS/LABA combination was associated with a significant decrease in the rate of exacerbations, similar, if not superior to the rate seen in the tiotropium group may support the use of the combination treatment rather than a long-acting beta-agonist alone.
The findings in this study regarding the effect of treatment on exacerbation frequency should be interpreted with caution as the protocol required the reporting of at least one exacerbation in the preceding year. Thus, 38.6% of the patients were of the frequent exacerbator phenotype, as defined by the current GOLD guideline. Patients who had experienced one exacerbation in the preceding year may have thus benefited less from the ICS/LABA combination. We have therefore examined our data according to the frequency of exacerbations in the previous year, as reported by the patients and found no evidence that the responses to either treatment were different between the frequent (two or more exacerbations) and infrequent exacerbators.
Another minor limitation is that the time of exacerbations during follow-up were recorded as the month when the patients reported them. A more precise account of the time (as the day of recurrence) might have allowed a more precise comparison of the time to the first exacerbation between the two groups. However, the patients were contacted monthly and there may have been issues of reliability as to the exact day of exacerbation reported by the patients.
We did not include a placebo arm in the study as both drugs were previously shown to be superior to placebo in similar patient populations and it would be unethical to withold these effective treatments from patients with moderate degrees of airflow obstruction (7,19).
In conclusion, tiotropium and salmeterol/fluticasone combination were found to provide similar improvements in the main clinical outcomes in COPD patients with moderate airfow obstruction. Because of the trends for differing effects in the rate of exacerbations and in oxygenation, further studies are warranted.
REFERENCES
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global initiative for chronic obstructive pulmonary disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55.
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Respir Res 2010;11:22-36.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. N Engl J Med 2010;363:1128-38.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten van M?lken MP, Beeh KM, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89.
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA; INSPIRE Investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26.
- Jenkins CR, Jones PW, Calverley PMA, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from randomized, placebo-controlled TORCH study. Respir Res 2009;10:59-67.
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;9:CD006829. doi:10.1002/14651858.CD006829.pub2.
- Sobieraj DM, White CM, Coleman CI. Benefits and risks of adjunctive inhaled corticosteroids in chronic obstructive pulmonary disease: a meta-analysis. Clin Ther 2008;30:1416-25.
- Miravitlles M, de la Roza C, Naberan K, Lamban M, Gobartt E, Martin A. (2007) Use of spirometry and patterns of prescribing in COPD in primary care. Respir Med 2007; 101:1753-60.
- Franssen FM, Spruit MA, Wouters EF. Determinants of polypharmacy and compliance with GOLD guidelines. Int J Chron Obstruct Pulmon Dis 2011;6:493-501.
- Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly 2010 Apr 21 (Epub ahead of print).
- Barnes NC, Qiu YS, Pavord ID, Parker D, Davis PA, Zhu J, et al; SCO30005 Stdy Group. Anti-inflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med 2006;173:736-43.
- Basyigit I, Yildiz F, Ozkara SK, Yildirim E, Boyaci H, Ilgazli A. Addition of inhaled corticosteroid on combined bronchodilator therapy in patients with COPD. Pulm Pharmacol Ther 2005;18:422-6.
- Adcock IM, Maneechotesuwan K, Usmani O. Molecular interactions between glucocorticoidsand long-acting beta2 agonists. J Allergy Clin Immunol 2002;110:S261-8.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54.
Yazışma Adresi (Address for Correspondence)
Dr. Pınar SARA?
Ege ?niversitesi Tıp Fak?ltesi,
G?ğ?s Hastalıkları Anabilim Dalı,
İZMİR - TURKEY
e-mail: ptaskiranlar@hotmail.com