RESEARCH ARTICLE
Doi: 10.5578/tt.22074
Tuberk Toraks 2016;64(2):97-104

Beş yıl boyunca allerjik olgularda eklenmiş omalizumab ve non-allerjik olgularda eklenmiş
oral steroid tedavilerinin sonu?ları
Bilun GEMİCİOĞLU1, Buket ?ALIŞKANER ?ZT?RK1, Berna DUMAN1
1 İstanbul ?niversitesi Cerrahpaşa Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, İstanbul, T?rkiye
1 Department of Chest Diseases, Faculty of Cerrahpasa Medicine, Istanbul University, Istanbul, Turkey
?ZET
Beş yıl boyunca allerjik olgularda eklenmiş omalizumab ve non-allerjik olgularda eklenmiş oral steroid tedavilerinin sonu?ları
Giriş: D?rd?nc? basamak astım tedavisi ile kontrol sağlanamayan allerjik olgularda eklenen omalizumab ve non-allerjik olgularda eklenen d?ş?k doz oral steroidin 5 yıl sonunda etkinliği ortaya konmak istenmiştir.
Hastalar ve Metod: D?rd?nc? basamak tedaviye rağmen kontrol sağlanamamış persitan astım olgularından 2006 ve 2014 yılları arasında en az beş yıl s?re ile izlenmiş olan omalizumab verilmiş allerjik veya d?ş?k doz oral steroid (minimum 4 mg, maksimum 12 mg, median 8 mg metil prednison) verilmiş non-allerjik olgular retrospektif olarak araştırmaya alınmıştır. Astım kontrol testi (AKT), FEV1 ve atak değerleri bu tedavilerin ?ncesinde (bazal) 1, 2 ve 5 yıl sonrasında yıllara g?re ve gruplar birbirleri ile karşılaştırılarak araştırılmıştır.
Bulgular: On yedi allerjik ve 16 non-allerjik olgu bulunmaktadır. Her iki grupta da bazale g?re 1, 2, 5. yıllarda anlamlı artış sağlanmıştır? (hepsi i?in p< 0.01). Allerjik grubun non-alerjik gruba g?re AKT değerlerinin 16. hafta, 1 ve 2. yıllarda d?zeldiği g?zlenmiştir (hepsi i?in p< 0.01). Ataklar non-alerjik grupta 5. yılda %39.6 (3.3 ortanca) oranında bazale (2.38 ortanca) g?re d?zeldiği g?zlenmiştir (p< 0.001). Allerjik grupta ise 5. yılda? bazale g?re %77.1 orananında daha az atak g?zlenmiştir (p< 0.001). Ancak buna rağmen allerjik grupta 5. yılda da olguların %47'sinde bir atak olduğu g?r?lm?şt?r.
Sonu?: Beşinci basamak tedavi olarak omalizumab eklenmesi kontrol ve ataklarda 5 yılda d?zelme sağlamış olsa da her iki grupta da ortalama olguların yarısının 5. yılda atak ge?irebildiği g?zlenmiştir.
Anahtar kelimeler: Astım kontrol?, astım tedavisi, astım atakları, omalizumab, oral steroid
SUMMARY
Comparison of allergic asthma patients treated with omalizumab and non-allergic patients treated with continuous oral corticosteroids: results of five year follow-up therapies
Introduction: To assess the long-term (5 year) efficacy of omalizumab and systemic corticosteroid therapy in allergic and non-allergic asthma that could not be controlled by step 4 therapy, respectively.
Patients and Methods: This single-center study was based on all consecutive step 4 patients with severe persistent uncontrolled allergic and non-allergic asthma who were given omalizumab and systemic corticosteroid (minimum: 4 mg, maximum: 12 mg, median: 8 mg methyl prednisone), respectively, in 2006-2014 and were followed up for at least 5 years. Asthma control test (ACT), FEV1 and exacerbation rates at initial presentation and 1, 2, and 5 years after step 5 treatment initiation were calculated for both groups.
Results: There were 17 and 16 allergic and non-allergic group patients, respectively. Both groups exhibited significant improvements in ACT at the 1st, 2nd, and 5th years relative to baseline (all p< 0.01). The allergic group had significantly better ACTs at 16 weeks, 1 year, and 2 years than the non-allergic group (all p< 0.01). By the 5th year, the exacerbations in the non-allergic group rose significantly by 39.6% (3.3 on average) compared to baseline (2.38 on average, p< 0.001). By contrast, the allergic group continued to have significantly fewer exacerbations at the 5th year (77.1% fewer relative to baseline, p< 0.001). However, 47% of the allergic group patients still presented with one exacerbation in the 5th year.
Conclusion: Adding omalizumab to step 5 therapy improved the control of severe persistent allergic asthma. However, nearly half of the patients in both groups presented at least one exacerbation in the 5th year.
Key words: Asthma control, asthma therapy, asthma exacerbations, omalizumab, oral corticosteroid
INTRODUCTION
The great majority of patients with severe asthma are step 4 asthmatics, meaning that their asthma can be controlled with high doses of inhaled corticosteroids and long acting β2 agonists. However, the remaining patients with severe asthma are candidates for step 5 treatment, as their condition cannot be controlled with step 4 medications (1,2,3). Since these patients have severe asthma, require many medications, frequently visit emergency rooms, and are often hospitalized, this condition places a significant burden on both the patients and the health care system (4,5).
Around 5-10% of patients with asthma have severe asthma. IgE-mediated immune mechanisms are thought to play a role in over 50% of these patients (6). Omalizumab is a recombinant humanized monoclonal antibody that complexes with free IgE, thereby preventing it from adhering to specific receptors on mast cells and basophils (FcRI). Omalizumab therefore acts as a potent anti-inflammatory agent in patients with severe perennial allergic asthma (7). Three studies with follow-up periods of 0.5, 4 and 7 years found that omalizumab effectively decreases asthma exacerbations, lowers steroid doses, and imposes better asthma control (8,9,10). Four randomized controlled studies of omalizumab also showed that it decreases asthma-related symptoms, reduces corticosteroid use, and improves quality of life (11,12,13,14).
In recent years, case reports and a Spanish multicenter registry study showed that omalizumab is also effective in non-allergic asthma (15). Thereafter, research efforts turned to assessing the usefulness of omalizumab in patients with non-allergic asthma. However, at present, the guidelines state that when non-allergic asthma is even unresponsive to macrolide or tiotropium medications and another diagnosis cannot be made, oral corticosteroid or anticytokine treatments should be administered (16,17). However, the long-term outcomes of patients with non-allergic step 5 asthma who do not accept treatment with continuous oral steroids remain unclear.
The present cohort study was performed to determine the outcomes over a 5 year follow-up period of patients whose severe persistent asthma was not controlled by step 4 treatment (ICS and LABA). If they had allergic asthma, omalizumab was added to the treatment. If they had non-allergic asthma, other controllers (but not continuous oral corticosteroid) were added. The two groups were examined for the rates of asthma control, asthma exacerbation, and other asthma variables at several time points over the 5 year follow-up period.
MATERIALS and METHODS
Selection of the Study Cohorts
The study protocol was approved by the local ethical board (no: 1643). The study was performed in the Department of Pulmonology of our university hospital, and the study period was 2006-2014. The study consisted of two groups of patients with severe persistent asthma that was not controlled by step 4 treatment. In all cases, asthma was diagnosed on the basis of GINA (Global Initiative for Asthma) criteria, namely, a clinical history of symptoms compatible with asthma and reversibility of bronchial obstruction, as measured by spirometry and defined as a post bronchodilator change in FEV1 (forced expiratory volume in one second) of 12% and 200 mL.
The allergic asthma group, was selected prospectively because its patients were enrolled in the November 2006 to September 2008 CIGE025A2425 international multicenter, open-label, parallel-group clinical trial, namely, the EXALT Study: randomized, open-label, parallel-group study to evaluate persistency of response to add-on omalizumab during 32 weeks' treatment (18). The inclusion criteria for the EXALT Study were as follows: all consecutive patients who were > 12 years old and had FEV1 < 80%, an asthma control test (ACT) < 20, at least two exacerbations that required treatment with systemic steroids in the 12 months before inclusion in the study, uncontrolled severe persistent asthma despite having regularly taken high-dose ICS and LABA, and smoking < 10 pack/year. The exclusion criteria for the EXALT Study were as follows: presence of any hematological, malignant, coronary, renal, hepatic, endocrinological, or gastrointestinal pathology that could affect the results, BMI > 35, steroid resistance, continuous oral steroid use, presence of mycoplasma or chlamydia saprophytes, or nocturnal, premenstrual, or brittle asthma. In total, 24 consecutive patients satisfied these eligibility criteria (n= 24). Of these, 17 were included in the EXALT Study because their total serum IgE levels were 30-700 IU/mL, they had perennial allergy (as established by a skin prick test and/or the presence of specific IgE), and they were suitable for omalizumab treatment (as indicated by the dose table, which was based on body weight and IgE levels). All patients enrolled in the EXALT Study started receiving omalizumab in addition to high-dose ICS and LABA. After the EXALT Study was completed in September 2008, these severe allergic asthma patients continued to receive omalizumab, high-dose ICS, and LABA, and were followed up for at least another 5 years.
The non-allergic group, was selected by a retrospective chart review that identified all consecutive patients with severe persistent non-allergic asthma that was initially not controlled by step 4 treatment and was then treated with step 5 therapy [combined high-dose inhaled steroid therapy and continuous oral corticosteroids: median 8 mg metyl prednisolone (minimum; 4 mg and maximum 12 mg)] for at least 5 years. The patients who met all of the eligibility criteria that were used to select the allergic asthma group (except positivity for the skin prick test) were enrolled in the present study. These patients were followed up between 2006 and 2014 in the outpatient clinic of the Department of Pulmonology.
Non adherence, inadequate inhalation technique,? nonstable comorbidities or persistent risk factors that interfere with the asthma control were also exclusion criterias during five years for all patients.
Primary Outcomes of Treatment
For both groups, the following information was gathered at several time points after the patients first presented with severe persistent asthma that was not controlled by step 4 therapy: (i) ACTs and FEV1 at the first presentation and 16 weeks, and at the end of 1, 2, and 5 years after presentation; (ii) number of exacerbations requiring at least 3 days of steroid treatment in the 12 months before step 5 therapy started, and the 1st, 2nd, and 5th year after step 5 commencement; (iii) amount of inhaled corticosteroid in the 12 months before step 5 therapy started, and 1 and 5 years after step 5 treatment started.
Statistical Analysis
The groups were compared in terms of the primary outcomes by using the SPSS 20.0 statistics program. Changes from the initial time point over time were assessed by student-t test. The two groups were compared by using the Mann-Whitney U test.
RESULTS?
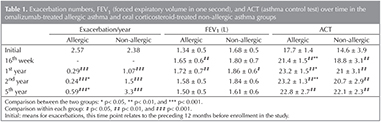
The allergic asthma group consisted of 17 patients (13 females and 4 males; average age, 48.3 ? 16.4 years). They had been followed up for 5.5 (range, 5.5-7) years. The non-allergic asthma group consisted of 16 patients (15 females and 1 male; average age, 52.3 ? 13.2 years). They had been followed up for 5.5 (range, 5.5-20) years. Table 1 indicates how each group changed over time in terms of number of exacerbations, FEV1, and ACT after step 5 therapy started. The table also shows how the two groups compared in terms of these variables at each follow-up time point. They have similar baseline ACTs, FEV1, and exacerbation rates (p> 0.05).
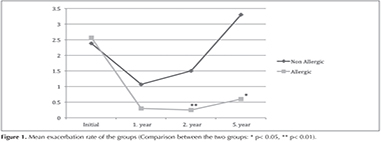
The mean exacerbation rate of two groups during 5 years is demonstrated in Figure 1.
The allergic and non-allergic groups had, on average, 2.57 and 2.38 exacerbations during the 12 months before the patients switched to step 5 therapy, respectively. By the end of the 1st and 2nd years, the exacerbations in both groups had dropped significantly compared to baseline (all p< 0.001). This drop continued to be observed in the allergic group at the end of the 5th year (0.59, p< 0.001 compared to baseline): this represented a 77.1% drop relative to baseline. By contrast, the number of exacerbations in the non-allergic group rose to 3.3 at the end of the 5th year (3.3, p< 0.001): this represented a 39.6% increase relative to baseline. Although the two groups did not differ significantly in terms of baseline exacerbation rates, the allergic group had significantly fewer exacerbations than the non-allergic group at the end of the 1st, 2nd, and 5th years (p< 0.02, p< 0.03, and p< 0.02, respectively). However, it should be noted that 8 of the 17 allergic group patients (47%) still had one or two exacerbations in the 5th year.
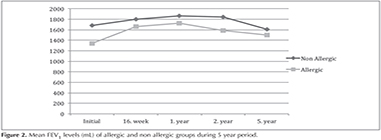
The baseline FEV1 levels of the non-allergic and allergic groups were 1.68 ? 0.5 L and 1.34 ? 0.5 L, respectively. The non-allergic group exhibited a significant increase in FEV1 at the 1 year time point only (p< 0.05), while the allergic group showed significant increases at both the 16th week and 1st year time points (p< 0.01 and p< 0.01, respectively). At the 5th year, the non-allergic group showed an 11% increase in FEV1, while the allergic group showed a 4.3% decrease in FEV1 relative to baseline; however, these changes relative to baseline were not statistically significant. While the 5th year average FEV1 was increased at 1.50 ? 0.5 L in the omalizumab group, this was not statistically significant. The two groups did not differ significantly at any time point in terms of FEV1 (p> 0.05). Despite the improvement seen in the allergic group, however, only 2 of these 17 (11.7%) patients reached FEV1 greater than 80% after 5 years of omalizumab therapy. Mean FEV1 levels of allergic and non allergic groups during 5 year period are seen on Figure 2.
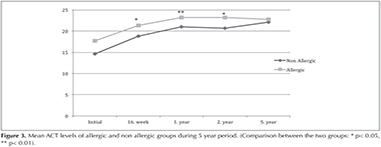
The baseline ACTs of the non-allergic and allergic groups were 14.6 ? 3.9 and 17.7 ? 1.4, respectfully. In both groups, the ACTs at all subsequent time points were significantly higher than at baseline (all p< 0.01). The allergic group had 13.5%, 10.16%, and 11.6% higher ACTs than the non-allergic group at the 16th week (p< 0.01), 1st year (p< 0.03), and 2nd year (p< 0.01), respectively. The allergic group also had a 3.1% increase in ACT at the 5th year compared to the non-allergic group, but this difference did not achieve statistical significance (p> 0.05). Only 3 patients (17.6%) in allergic group and 2 patients (12.5%) in non allergic group had ACT below 20 at the end of the 5th year. Mean ACT levels of allergic and non allergic groups during 5 year period are seen on Figure 3.
The inhaled steroid dosage of the non-allergic group did not change over time relative to baseline (p> 0.05). By contrast, the inhaled steroid dosage of the allergic group dropped after starting omalizumab therapy: compared to the inhaled steroid dosage in the 12 months preceding the start of step 5 therapy, a drop of 65% was observed at the 5th year. The allergic group also exhibited significantly lower inhaled steroid dosages than the non-allergic group at the 1st and 5th year (p< 0.002 and p< 0.002, respectively), even though the two groups did not differ significantly at baseline in terms of this variable. Despite the improvement seen in the allergic group, however, 6 of these 17 (35%) patients still required high doses of inhaled steroid after 5 years of omalizumab therapy.
DISCUSSION
Although we have some limitations of the study regarding the small number of patients in the study groups and the retrospective analysis of the non allergic groups, we showed that the allergic group, who received omalizumab, generally performed quite well over the 5 year treatment. Thus, compared to the 12 months before step 5 therapy started, the exacerbations in the allergic group had dropped by 77.1% by the end of the fifth year. However as an unexpected result of the study, 47% of the patients presented at least one excerbation in the fifth year.? The allergic group also exhibited a rise in ACTs by 31% at the fifth year, although this did not achieve statistical significance. Only %17.6 had ACT below 20. The contrast between the exacerbation rate end the results of the ACT in the fifth year is attributed to the methodology is one of the limitation of the study. As ACT results reflect only the last month and were taken at the end of the each treatment year in our study, if the exacerbation was in an other time of the year patient might be better after some months.? The problem may be the drop in the dosage of inhaled steroid as the allergic group showed a 65% drop in inhaled steroid dosage at the fifth year.
Several studies showed that omalizumab is an effective and safe treatment for severe allergic asthma.In the German registry real life study, omalizumab treatment decreased exacerbations by 75% at the 16th week (19). Real-life data from 14 countries showed that 2 year of omalizumab treatment significantly improved the exacerbation rate, by 67.3% (20). The exacerbation rate decreased 70% in the Southeastern mediterannean real life study after 4 years (21). Our present study showed that omalizumab treatment decreased exacerbations in the allergic asthma group by 77.1% at the fifth year.
Bousquet et al. pooled the data of seven studies, they found that omalizumab decreased asthma exacerbations in patients with severe allergic asthma by 38% (22). Hanania et al study in 850 patients, showed that omalizumab treatment decreased 25% more exacerbations then plasebo after 48 weeks (23). The systematic review of Norman et al. also showed that in patients with allergic asthma, omalizumab decreased the exacerbations (24). A systematic meta-analysis evaluating the effectiveness of omalizumab versus placebo in 3429 patients with moderate-severe persistent allergic asthma found that omalizumab treatment (1883 patients) significantly improved asthma exacerbations, steroid dose requirement, and asthma symptom scores (25).
The EXELS study published in 2012 showed that omalizumab treatment significantly improved ACTs in allergic asthma patients at the sixth month and that this remained evident at the second year of follow-up (26). They showed that at the end of the second year 59% of patient had ACT ≥ 20. This rate was 82.3 at the end of the fifth year in our study. Ozgur ES et al from Turkey showed that long-term therapy with omalizumab for up to 3 years significantly increased ACT (27). Similarly, the present study showed that omalizumab significantly improved ACTs at? the 16th week (13.5% improvement relative to baseline) and that this effect persisted at the first (10.6%), second (11.9%), and fifth (3.1%) year.
With regard to dosage of inhaled corticosteroids, three studies in 2001 found that omalizumab decreased the need for inhaled corticosteroids (28,29,30). A Spanish study by Vennera et al. also showed that after a 2 year follow-up, the required steroid dosages of patients with severe allergic asthma decreased after omalizumab therapy (31). Similarly, the present study showed that omalizumab significantly decreased the inhaled steroid dosage of the allergic asthma group by 65% at the fifth year.
While the 5 year omalizumab treatment did improve most asthma variables in the patients with allergic asthma, 47% still had at least one exacerbation in the fifth year. Moreover, 35% still required high doses of inhaled steroid therapy, and 88% had FEV1 below 80% at the fifth year. We believe that those problems still must be questionned in regard to need of other therapies.
Regarding the non-allergic group in the present study, the fact that exacerbations rose by 39.6% relative to baseline at the fifth year contrasts with the fact that the same group consistently exhibited significant increases in ACT, even at the fifth year. This may be attributed to the good adherence of these patients to step 5 therapy due to their routine follow-up in a university hospital outpatient clinic and their expectation of receiving a new drug for asthma. However, this is speculation at present: we did not examine whether non-drug factors such as treatment adherence could be responsible for the increased ACT in this group (1). The Spanish registry study of Llana et al. showed that during a 2 year period, omalizumab also significantly improved the ACTs of non-allergic asthma patients at the 1st and 2nd years. However, it had no effect on FEV1 and exacerbations (15). In the present study, the step 5-treated non-allergic group, who were not treated with omalizumab, also exhibited significantly increased ACTs at all time points. However, unlike the Spanish study, the non-allergic group exhibited significant decreases in exacerbation rates at all time points as well. With regard to FEV1 levels, there was a small increase in this variable in the omalizumab group (11%) at the end of the fifth year and a small decrease (4.3%) in the non-allergic group. These changes relative to baseline did not achieve statistical significance.
CONCLUSION
The present study showed that in suitable patients whose asthma is difficult to control, omalizumab can effectively improve asthma control and prevent exacerbations. However, many of the omalizumab-treated allergic asthma patients still exhibited exacerbations. This was also observed for the step 5-treated non-allergic patients. Especially, non allergic group of patients demonstrated a need for new medications paves the way for improving the treatment of non-allergic asthma patients.
REFERENCES
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2015. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_2015_Aug11.pdf
- Yildiz F, Oguzulgen IK, Dursun B, Mungan D, Gemicioglu B, Yorgancioglu A; TTS Asthma and Allergy Working Group Guideline Committee for Asthma. Turkish Thoracic Society asthma management and prevention guideline: key points. Tuberk Toraks 2011;59:291-311.
- Expert Panel Report3 (EPR-3) Guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(5 Suppl 1):94-138.
- Bavbek S, Misirligil Z; Study Group. A breath for health: an explatory study in severe asthma patients in Turkey. Allergy 2008;63:1218-27.
- Celik GE, Bavbek S, Pasaoglu G, Mungan D, Abado?lu O, Harmanci E, et al. Direct medical cost of asthma in Ankara, Turkey. Respiration 2004;71:587-93.
- The ENFUMOSA Study Group. The ENFUMOSA cross sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J 2003;22:470-7.
- Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008;8:218-30.
- Humbert M, Beasley R, Ayres J, Slavin R, H?bert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are in adequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16.
- Pace E, Ferraro M, Bruno A, Chiappara G, Bousquet J, Gjomarkaj M. Clinical benefits of 7 years of treatment with omalizumab in severe uncontrolled asthmatics. J Asthma 2011;48:387-92.
- Menzella F, Facciolongo N, Piro R, Formisano D, Roggeri A, Simonazzi A, et al. Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Respir Dis 2012;6:87-95.
- Buhl R, Hanf G, Sol?r M, Bensch G, Wolfe J, Everhard F, et al. The anti-IgE antibody omalizumab improves asthma- related quality of life in patients with allergic asthma. Eur Respir J 2002;20:1088-94.
- Buhl R, Sol?r M, Matz J, Townley R, O'Brien J, Noga O, et al. Omalizumab provides long- term control in patients with moderate-to-severe allergic asthma. Eur Respir J 2002;20:73-8.
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy 2004;59:7018.
- Deniz Y, Gupta N. Safety and tolerability of omalizumab (Xolair), a recombinant humanized monoclonal anti-IgE antibody. Clin Reviews Allergy Immunol 2005;29:31-48.
- De Llano LP, Vennera Mdel C, ?lvarez FJ, Medina JF, Border?as L, Pellicer C, et al; Spanish Registry. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma 2013;50:296-301.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73.
- Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013;368:2455-66.
- Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011;66:671-8.
- Schumann C, Kropf C, Wibmer T, R?diger S, Stoiber KM, Thielen A, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J 2012;6:215-27.
- Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The experience registry: the "real-world" effectiveness of omalizumab in allergic asthma. Respir Med 2013;107:1141-51.
- Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean "real-life" experience. Pulm Pharmacol Ther 2012;25:77-82.
- Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 2005;60:302-8.
- Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011;154:573-82.
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 2013;17:1-342.
- Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 2011;139:28-35.
- Eisner MD, Zazzali JL, Miller MK, Bradley MS, Schatz M. Longitudinal changes in asthma control with omalizumab: 2-year interim data from the EXCELS Study. J Asthma 2012;49:642-8.
- Ozgur ES, Ozge C, Ilvan A, Nayci SA. Assessment of long-term omalizumab treatment in patients with severe allergic asthma long-term omalizumab treatment in severe asthma. J Asthma 2013;50:687-94.
- Sol?r M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001;18:254-61.
- Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001;108:E36.
- Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med 2009;103:1725-31.
- Vennera Mdel, P?rez De Llano L, Bardag? S, Ausin P, Sanjuas C, Gonz?lez H, et al; Omalizumab therapy in severe asthma: experience from the Spanish registry-some new approaches. J Asthma 2012;49:416-22.
Yazışma Adresi (Address for Correspondence)
Dr. Bilun GEMİCİOĞLU
İstanbul ?niversitesi Cerrahpaşa Tıp Fak?ltesi,
G?ğ?s Hastalıkları Anabilim Dalı,
İSTANBUL - TURKEY
e-mail: bilung@gmail.com