RESEARCH ARTICLE
Doi: 10.5578/tt.9478
Tuberk Toraks 2016;64(1):53-59

İntratorasik lenfadenopatilerin tanısında transbronşiyal iğne aspirasyonu y?ntemi ile
elde edilen aspirata sitopatolojik yaklaşım
G?lzade ?ZYALVA?LI1, Zehra YAŞAR2, Erdoğan ?ETİNKAYA3
1 Abant İzzet Baysal ?niversitesi Tıp Fak?ltesi, Tıbbi Patoloji Anabilim Dalı, Bolu, T?rkiye
1 Department of Medical Pathology, Faculty of Medicine, Abant İzzet Baysal University, Bolu, Turkey
2 Abant İzzet Baysal ?niversitesi Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, Bolu, T?rkiye
2 Department of Chest Diseases, Faculty of Medicine, Abant İzzet Baysal University, Bolu, Turkey
3 Yedikule G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği, İstanbul, T?rkiye
3 Clinic of Chest Diseases, Yedikule Chest Diseases and Chest Surgery Training and Resarch Hospital, İstanbul, Turkey
?ZET
İntratorasik lenfadenopatilerin tanısında transbronşiyal iğne aspirasyonu y?ntemi ile elde edilen aspirata sitopatolojik yaklaşım
Transbronşiyal iğne aspirasyonu (TBİA), mediastinel lenf nodu ve peribronşiyal lezyonların ?rneklemesine imkan veren etkili, g?venli ve ucuz bir y?ntemdir. TBİA, bronkojenik karsinom evrelemesinde, peribronşiyal ve submukozal lezyonlarda, sarkoidoz ve t?berk?lozun tanısında, t?m?r?n submukozal yayılımının ayırt edilmesinde, mediastinal kitlelerin tanısında kullanılır. Bu derlemede, Abant İzzet Baysal ?niversitesindeki deneyimlerimizden faydalanarak, intratorasik lenfadenopatilerde TBİA y?nteminin tanısal başarısının arttırılabilmesi i?in elde edilen aspirasyon materyalinin yeterliliği, sitopatolojik-histopatolojik değerlendirilmesi ve ayırıcı tanısı ele alınmıştır.
Anahtar kelimeler: Sitoloji, respiratuar aspirasyon
SUMMARY
A cytopathological approach to diagnosing intrathoracic lymphadenopathy using aspirates obtained by the transbronchial needle aspiration method
Transbronchial needle aspiration (TBNA) is an effective, safe and cost-effective technique that allows for sampling of the mediastinal lymph node and peribronchial lesions. It is used in bronchogenic carcinoma staging, peribronchial and submucosal lesions, diagnosis of sarcoidosis and tuberculosis, differentiating submucosal invasion, and in diagnosing mediastinal masses. From our experience at the University of Abant Izzet Baysal and from a review of the literature, we discuss the adequacy and the differential diagnosis of aspiration material obtained by TBNA and cytopathological-histopathological evaluation in intrathoracic lymphadenopathies to increase the success rate of the TBNA method.
Key words: Cytology, respiratory aspiration
Geliş Tarihi/Received: 07.04.2015 • Kabul Ediliş Tarihi/Accepted: 14.04.2015
INTRODUCTION
Lymphadenopathy (LAP) occurs after a response of the lymph node to an exogenous or endogenous agent. Microorganisms, autoimmune diseases, immune insufficiencies, foreign bodies and tumors are all possible reasons for development of lymphadenopathy (1).? Since the mediastinum has a rich lymph node and lymphatic network, there is extensive lymph flow to the lymph nodes from various organs in the mediastinum, neck, and lower diaphragm. Intrathoracic lymph nodes may be affected by localized inflammatory disease, primary lymphatic tumors, and pathologies originating from the chest wall, breast or a remote organ. Non-invasive and invasive methods are used to diagnose mediastinal and hilar lymphadenopathy. Although invasive procedures such as mediastinoscopy are the gold standard in determining the etiology of, and staging the malignancy of, mediastinal lymphadenopathies, the diagnostic range of transbronchial needle aspiration (TBNA) is increased by the addition of methods such as endobronchial ultrasonography (EBUS).
TBNA is used in bronchogenic carcinoma staging, in peribronchial and submucosal lesions, peripheral nodule and masses, endobronchial lesions, sarcoidosis and tuberculosis, in differentiating sub mucosal invasion, and in diagnosing mediastinal masses. EBUS-TBNA, which is an effective method of extending the bronchoscopist's field of view beyond the bronchial lumen, is still not widely used because expensive equipment is required (2,3). If used by experienced physicians and with sufficient sampling, TBNA, which is an effective, safe and cheap bronchoscopy technique, may reduce the need for invasive procedures in the diagnosis of mediastinal or hilar lymphadenopathy, and the staging of lung cancer (4,5,6).
In this review, the adequacy, cytopathological-histopathological evaluation, and differential diagnosis of the aspiration material obtained by TBNA in intrathoracic lymphadenopathies are evaluated to increase the success rate of the TBNA method.
TRANSBRONCHIAL NEEDLE ASPIRATION (TBNA)
Transbronchial needle aspiration is a technique for performing cytological, histological or microbiological sampling of lesions within mediastinal pathologies adjacent to the tracheobronchial tree, trachea or airway wall, and lung parenchyma (7). Today, TBNA can be performed with conventional BT (from where the external compression been detected or according to Wang's map), electromagnetic guidance, and with ultrasound guidance (radial probe or convex robe).
Factors Affecting the Success of TBNA
Presence of lymph node in BT: Sensitivity is higher in patients with lymph node enlargement in BT (lymph node larger than 1 cm).
Needle type: The histology needles introduced by Wang et al. for diagnosing cancer have increased sensitivity. In comparison studies, 19-, 21-, and 22- gauge needles were evaluated; a 19-gauge histology needle was found to have a significantly higher success rate (8).
Lymph node localization: Lymph node localization affects the positivity of TBNA results. When similar studies in the literature were reviewed, it was found that the sensitivity of TBNA was higher in left-sided compared to right-sided tumors, and in right paratracheal and subcranial lymph node aspirates compared to left paratracheal aspirates (9).
Size of lymph node: Tumor-positive aspirates have been shown to increase linearly with a lymph node size from 1 cm to 2-2.5 cm (9).
Number of aspirations from lymph node station: Studies recommend at least four aspiration samplings from each lymph node station to obtain sufficient material, and state that seven samplings would increase the ratio of diagnosis (10).
Rapid onsite cytopathology presence: This procedure, which is known as rapid on-site evaluation (ROSE), increases the ratio of diagnosis for TBNA as it allows on-site evaluation by a pathologist (11).
User experience: Studies indicate that performing nearly 50 procedures is sufficient for diagnostic efficiency (12).
Ultrasound-guided TBNA: In a study in which 200 patients were randomized to an EBUS-guided and a conventional TBNA, EBUS-guidance significantly increased the yield of TBNA (from 58% to 84%) in all stations, with the exception of the subcarinal region (from 74% to 86%) (13).
OBTAINING ADEQUATE-QUALITY AND SUFFICIENT MATERIAL IN TBNA
Obtaining adequate quality and a sufficient quantity of material with the TBNA method depends on the communication between the clinician and the pathologist (5). ROSE is used to examine the adequacy of the aspirates macroscopically and microscopically by a pathologist. This method helps to preventing adequate sampling. The experience of the clinician and evaluation by the pathologist on site increase the diagnostic value of the method (5).
The histological tissue obtained by these method is fixed in a mixture of 50% formaldehyde and 50% ethyl alcohol to form a cell block. The fixation method for the sampled cytological material obtained using a fine needle should be specified by the pathologist. If the pathologist prefers to evaluate the aspirate by Papanicolaou staining, a thin layer of the material should be spread on a glass slide and immediately immersed in 95% ethyl alcohol or fixed with spray fixatives. If the material does not come into contact with the fixative within 10 seconds, artifacts due to drying may occur, which could lead to an insufficient and suspicious diagnosis. If May-Gr?nwald-Giemsa staining is used in cytological evaluation, the glass slides should be dried rapidly (14).
Adequacy Criteria
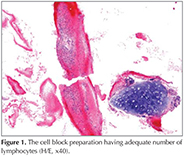
The criteria related to the adequacy of the aspiration material in TBNA are unclear and vary considerably among observers. The number of lymphocytes is of great importance in detecting the adequacy of the obtained material. Adequate material is defined as the presence of 40 benign lymphocytes in a high-powered field in the areas with the greatest cellularity (Figure 1) (5). Some authors have reported that at least 30% of the cells should be lymphocytes to have adequate material (15). If granulomatous structures or malignant cells are present, the material obtained is considered to be adequate even if there are few lymphocytes. Similarly, the presence of macrophages containing a large number of anthracotic pigments, together with abundant lymphocytes and pigmented macrophages, indicates that the material is adequate (16).
A polymorphous lymphoid population with tingible body macrophages is possibly indicative of an aspirate from a reactive lymph node (5). The presence of only endobronchial material (ciliated pseudostratified prismatic epithelium, mucus) without any lymphocytes in the aspirate spread over a glass slide shows that the material is not adequate. Such aspirates should not be regarded as "benign" (17). Sometimes, changes in contaminated endobronchial cells such as metaplasia or dysplasia may be misinterpreted and cause false positive results (5). If the aspirate contains numerous malignant cells without any lymphocytes, one should consider the possibility that the material may have been taken from the primary tumor (5).
CYTOPATHOLOGICAL-HISTOPATHOLOGICAL DIFFERENTIAL DIAGNOSIS OF INTRATHORACIC LYMPHADENOPATHIES WITH TBIA
Factors Causing Granulomatous Inflammation
Tuberculosis: Because tuberculosis is a common disease in our country, differentiation of tuberculosis is essential in TBNA. Granulomas are recognized by the loosely cohesive nature of epithelioid histiocytes in the presence of lymphocytes (5). Granulomas may be formed by 10 to over 100 histiocytes with bean-shaped, spindled, twisted or boomerang-shaped nuclei, and abundant pale-staining cytoplasm with ill-defined borders (18). Granulomas can often be misdiagnosed as sarcoidosis. Tuberculosis causes chronic granulomatous inflammation with caseification necrosis. On the other hand, granulomas in sarcoidosis are not necroptic. However, granulomas in tuberculosis are not always present with necrosis, and thus the pathological diagnosis of the presence of granulomas without necrosis in the aspirate should be "chronic granulomatous inflammation without necrosis", and it should be a noted that "The patient is recommended to be clinically and microbiologically evaluated for granulomatous diseases". Before definitive diagnosis, clinical and microbiological findings should be studied, and microbiological culture and acid-fast bacillus staining should be performed on the aspiration material obtained by TBNA (19). Similarly, despite having a low diagnostic value, Ziehl-Neelsen staining should be performed on the cell block for acid-fast bacilli. If needed, a PCR method can also be employed (20). In a study conducted by Cetinkaya et al. on intrathoracic lymphadenopathy patients to evaluate the TBNA method, it was concluded that TBNA was a valuable tool in the diagnosis of tuberculosis and sarcoidosis and should be considered before other invasive methods (21).
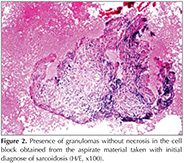
Sarcoidosis: Sarcoidosis is a disease of unknown etiology that is characterized by non-necrotic granulomas. It is observed mostly in parenchymal infiltration and hilar lymphadenopathies (22,23). There may be systemic involvement that includes organs such as the eyes and skin, and the disease may even lead to cardiac and neurological function loss in some patients (20,24). Along with the clinical and radiological findings, sarcoidosis is diagnosed when noncaseating granulomas are present or the cytological materials contain epithelioid histiocytes without necrosis (20). However, factors causing other granulomatous diseases-such as tuberculosis and fungal infections-should be excluded by clinical and microbiological evaluation (20,23). As the ratio of hilar and mediastinal involvement is > 85%, granulomas are usually detected by bronchoscopic methods (25). In fine-needle aspiration material, sarcoidosis granulomas are typically tightly packed, and they may present as numerous histiocytic aggregates without necrosis (Figure 2) (5).
Fungal infection: Fungal infections may present as granulomatous Inflammation in TBNA. In particular, granulomatous inflammation from a blastomycosis origin presents as a suppurative granuloma containing neutrophils in cytopathologic and/or histopathologic evaluation (26). There may be varying amounts of necrosis accompanied by neutrophils or pyknotic ghost cells in the granular layer. Walls of the fungal organism can be visualized most effectively using a microscope at low condendation. If the ROSE method reveals a granuloma, culture should be performed to test for fungal or mycobacterial infection. If a fungal organism is suspected in the cell-block material, use of specific stains-such as PAS, GMS and musicarmen-facilitates diagnosis and type classification (5).
Malignant epithelial tumors: Sarcoidosis-like granulomas may be present in lymph nodes that are draining malignant cells. Methods employed for staging in non-small-cell lung carcinomas revealed that 4% of mediastinal lymph nodes contained sarcoid-like granulomas (27). Moreover in the presence of a metastatic keratinizing squamous cell carcinoma, a foreign body type granulomatous reaction can be observed in the lymph node, and granulomas may accompany other malignancies (28). By taking into account the fact that granulomas can accompany various malignancies, cytopathologists should not immediately interpret the presence of granuloma in TBNA aspirate as a benign cytology (sarcoidosis, tuberculosis) but perform additional imaging methods to exclude malignancy (5).
Situations or Cells in the Aspiration Material That May Lead to False-Positive Results for Malignancy
Occasionally, the aspirate may contain a large number of contaminating endobronchial cells or bronchial material (cartilage). These cells should be carefully evaluated in terms of criteria for atypical cells. Reactive or dysplastic changes in endobronchial cells should not be immediately interpreted as a malignancy since doing so may give a false-positive result.? In particular, benign mesothelial cells may be present in the aspiration material of patients with pleural effusion. Differentiation should be performed with great care due to atypical glandular cells that can be observed in patients receiving chemotherapy-radiotherapy, or in squamous metaplasia, which is a frequent condition in endobronchial cells that can occur as a side effect of cigarette smoke (5). Rarely, benign mesothelial cells can be observed; this is particularly common in aspirates taken from patients with pleural effusion. Mesothelial cells, presenting intracellular "windows", have a wide cytoplasm, low nucleus to cytoplasm ratio, a bland nucleus, and a scalloped border. Generally, they are in the form of a single cell or small aggregates in the aspirate material. If a cell block is obtained, it is easier to differentiate these cells from other malignant cells by an immunohistochemical method (29).
Primary Lung Tumors
Small-cell lung carcinoma: The first step when an aspirate material has been judged to be definitely malignant is to differentiate metastasis and primary cancer, and determine whether it is a small-cell or non-small-cell carcinoma (NSCC) when the tumor originates in the lung. In small-cell lung carcinomas, the aspiration material is usually cellular, contains varying amounts of necrosis, and composes of single or loosely attached cells that have high nucleus cytoplasm ratio, round to oval hyperchromatic nucleus, scant cytoplasm and inconspicuous nucleoli. Molding and crush artifacts in tumor cells are the prominent features. It can be difficult to differentiate squamous cell carcinomas from other variants, especially from basal squamous cell carcinomas and other non-small-cell lung carcinomas. In such cases, immunohistochemical staining of the cell block is helpful. Small-cell carcinomas are positive for at least two neuroendocrine markers (chromogranin, synaptophysin, CD56, neuron specific enolase) and weakly positive for thyroid transcription factor 1 (TTF-1). On the contrary, in squamous cell carcinomas, tumor cells are positive for p40, p63 and high-molecular-weight cytokeratin (for example CK 5/6) (30,31).
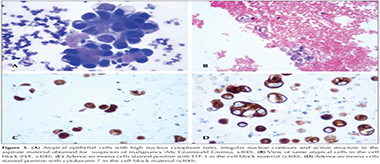
Non-small-cell lung carcinoma: Squamous cell carcinomas, adenocarcinomas, and large-cell carcinomas are known as non-small-cell carcinomas (NSCC). Cytoplasmic keratinization, desmosomes, hyperchromatic nuclei with unclear nuclear details, and the presence of many unclear nucleoli are the typical features of squamous cell carcinomas. Glandular differentiation, cytoplasmic vacuoles, vesicular nuclei, and marked nucleoli are the classical features of adenocarcinomas (Figure 3a, Figure 3b). However, diagnosis of poorly differentiated NSCC tumors may be difficult. Although adenocarcinomas have a poor prognosis, they respond well to target-oriented treatment (EGFR). In squamous cell carcinomas treated with bevacizumab, an endothelial growth factor inhibitor, differentiation should be made between squamous cell carcinomas and adenocarcinomas, as there is a possibility of fatal pulmonary hemorrhage? (32). Mucin stationing of the cell block (mucicarmine, diastase PAS) is rarely positive for adenocarcinomas. If mucin staining is negative, immunohistochemical application of CK5/6, CK7, p40, p63, Napsin A and TTF-1 can facilitate differentiation. Adenocarcinomas are usually positive for TTF-1, Napsin A and CK7 while squamous cell carcinomas are positive for p40, p63 and high-molecular-weight cytokeratin (Figure 3c, Figure 3d) (5,31). However, not all adenocarcinomas are positively stained with TTF-1. Only 60-70% of high-grade adenocarcinomas show positive staining for TTF-1. Moreover, some extrapulmonary adenocarcinomas (endometrial, ovarian, and colorectal) can occasionally show positive staining with TTF-1 (33). There may be extensive expression of p40 and p63 in all squamous cell carcinomas, including basaloid variants, irrespective of the grade. If there is no expression of TTF-1 or p40 mucin is negative, and keratinization is unclear, such tumors should be reported as NSCC-NOS (5).
Metastatic Tumor
If there is no extrathoracic malignancy history known to the clinician, differentiation of primary and metastatic tumors can be difficult. Renal cell carcinoma is not a frequently encountered tumor. If the patient has a history of extrathoracic tumor, an immunohistochemical panel (prostate specific antigen, trioglobulin, HepPar l and similar) for CK7 and CK20 is recommended to differentiate primary tumor and metastasis (33).
Lymphoma
Sometimes, atypia of lymphocytes may go unnoticed while searching for granulomas in the aspirate. Small-large lymphocytes, lymphohistiocytic aggregates, and tingible-body macrophages generally represent a reactive lymph node. However, a lymphoma should be suspected in the presence of a small or large monotonous population, or atypically large lymphoid cells. In the ROSE method, it is important to obtain a sample adequate for performance of flow cytometry for immune phenotyping if atypical large lymphoid cells are remarkable or the patient has a history of lymphoma. However, if flow cytometry cannot be performed, the type of lymphoma can be determined by immunohistochemical staining of the cell block (34). In preparations that do not allow for optimal evaluation, differential diagnosis should be carefully made by taking into account tumors mimicking a lymphoma, such as carcinoid tumors and small-cell carcinomas, and attention should be paid to differentiating between atypical lymphocytes observed in lymphomas and atypical epithelial cells observed in carcinomas (35,36).
In conclusion, TBNA, used in diagnosing and staging of lung carcinomas, is a minimally invasive method that facilitates collection of cytology samples, and is recommended for use in patients suspected to have mediastinal and hilar lymph involvement. Obtaining adequate aspiration material and evaluation of these samples by an experienced pathologist increases the percentage of correct diagnoses. Close cooperation between the clinician and the pathologist is important to increase the rate of correct diagnosis when using TBNA.
REFERENCES
- Ioachim HL, Medeiros LS. Ioachim's Lymph Node Pathology. 4th edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008.
- Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92.
- Yasufuku K, Nakajima T, Chiyo M, Sekine Y, Shibuya K, Fujisawa T. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol 2007;2:970-9.
- DeMay RM. Lung. In: the art and science of cytopathology.Volume 2. Chicago: ASCP Press, 1996:947-98.
- Cameron SEH, Andrade RS, Pambuccian SE. Endobronchial ultrasound-guided transbronchial needle aspiration cytology: a state of the art review. Cytopathology 2010;21:6-26.
- Bonifazi M, Zuccatosta L, Trisolini R, Moja L, Gasparini S. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration 2013;86: 123-34.
- Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration? biopsy of paratracheal tumors. Am Rev Respir Dis 1978;118:17-21.
- Schenk DA, Chambers SL, Derdak S, Komadina KH, Pickard JS, Strollo PJ, et al. Comparison of the Wang 19-gauge and 22-gauge needles in the mediastinal staging of lung cancer. Am Rev Respir Dis 1993;147:1251-8.
- Harrow EM, Abi-Saleh W, Blum J, Harkin T, Gasparini S, Addrizzo Harris DJ, et al. The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med 2000;161:601-7.
- Chin R Jr, McCain TW, Lucia MA, Cappellari JO, Adair NE, Lavato JF, et al. Transbronchial needle aspiration in diagnosing and staging lung cancer: how many aspirates are needed? Am J Respir Crit Care Med 2002;166:377-81.
- Diette GB, White P Jr, Terry P, Jenckes M, Rosenthal D, Rubin HR. Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest 2000;117:1186-90.
- Raveglia F, Meda S, Conforti S, Leporati A, Calati AM, Squinzi R, et al. Diagnostic value and learning curve of transbronchial needle aspiration in thoracic surgery. Minerva Chir 2006;61:459-66.
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5.
- ?nal B. Histopatolojik ve sitopatolojik tanı y?ntemleri. T?rkiye Klinikleri J Int Med Sci 2006;2:57-73.
- Patelli M, Lazzari Agli L, Poletti V, Trisolini R, Cancellieri A, Lacava N, et al. Role of fiberscopic transbronchial needle aspiration in the staging of N2 disease due to non-small cell lung cancer. Ann Thorac Surg 2002;73:407-11.
- Alsharif M, Andrade RS, Groth SS, Stelow EB, Pambuccian SE. Endobronchial ultrasound-guided transbronchial fine-needle aspiration: the University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol 2008;130:434-43.
- Cappellari JO, Haponik EF. Bronchoscopic needle aspiration biopsy. Am J Clin Pathol 2000;113(Suppl 1):S97-S108.
- Fritscher-Ravens A, Sriram PV, Topalidis T, Hauber HP, Meyer A, Soehendra N, et al. Diagnosing sarcoidosis using endosonography-guided fine needle aspiration. Chest 2000;118:928-35.
- Sel?uk ZT. Transbronşiyal iğne aspirasyonu. In: Metintaş M (ed.). Bronkoskopi. Ankara: Poyraz Tıbbi Yayıncılık, 2008;5;217-34.
- Nakajima T, Yasufuku K, Kurosu K, Takiguchi Y, Fujiwara T, Chiyo M, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis e comparisons with other bronchoscopic diagnostic modalities. Respir Med 2009;103:1796-800.
- ?etinkaya E, Yıldız P, Kadakal F, Tekin A, Soysal F, Elibol S, et al. Transbronchial needle aspiration in the diagnosis of intrathoracic lymphadenopathy. Respiration 2002;69:335-8.
- Miliauskas S, ?emaitis M, Sakalauskas R. Sarcoidosis- moving to the new standard of diagnosis? Medicina (Kaunas) 2010;46:443-6.
- Agarwal R, Aggarwal AN, Gupta D. Efficacy and safety of conventional transbronchial needle aspiration in sarcoidosis: a systematic review and meta-analysis. Respir Care 2013;58:683-93.
- Tigin HC, Kıyık M, Mutlu N, Artan E, Karadeli T, Durmaz A ve ark. Pulmoner sarkoidozda tanı y?ntemleri. Solunum 2008;10:85-8.
- Chapman JT, Mehta AC. Bronchoscopy in sarcoidosis: diagnostic and therapeutic interventions. Curr Opin Pulm Med 2003;9:402-7.
- El-Zammar OA, Katzenstein AL. Pathological diagnosis of granulomatous lung disease: a review. Histopathology 2007;50:289-310.
- Steinfort DP, Irving LB. Sarcoidal reactions in regional lymph nodes of patients with non-small cell lung cancer: incidence and implications for minimally invasive staging with endobronchial ultrasound. Lung Cancer 2009;66:305-8.
- Khurana KK, Stanley MW, Powers CN, Pitman MB. Aspiration cytology of malignant neoplasms associated with granulomas and granuloma-like features: diagnostic dilemmas Cancer 1998;84:84-91.
- Paull G, Mosunjac M. Fine-needle aspiration biopsy and intraoperative cytologic smear findings in a case of benign mesothelial-cell inclusions involving a lymph node: case report and review of the literature. Diagn Cytopathol 2003;29:163-6.
- Aslan DL, Gulbahce HE, Pambuccian SE, Manivel JC, Jessurun J. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 2005;123:874-8.
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al.; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60.
- Rossi G, Pelosi G, Graziano P, Barbareschi M, Papotti M. A reevaluation of the clinical significance of histological subtyping of non--small-cell lung carcinoma: diagnostic algorithms in the era of personalized treatments. Int J Surg Pathol 2009;17:206-18.
- Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med 2008;132:384-96.
- Kennedy MP, Jimenez CA, Bruzzi JF, Mhatre AD, Lei X, Giles FJ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5.
- De Las Casas LE, Gokden M, Mukunyadzi P, White P, Baker SJ, Hermonat PL, et al. A morphologic and statistical comparative study of small-cell carcinoma and non-Hodgkin's lymphoma in fine-needle aspiration biopsy material from lymph nodes. Diagn Cytopathol 2004;31:229-34.
- Geisinger KR. Differential diagnostic considerations and potential pitfalls in fine-needle aspiration biopsies of the mediastinum. Diagn Cytopathol 1995;13:436-42.
Yazışma Adresi (Address for Correspondence)
Dr. G?lzade ?zyalva?lI
Abant İzzet Baysal ?niversitesi Tıp Fak?ltesi,
Tıbbi Patoloji Anabilim Dalı,
BOLU - TURKEY
e-mail: gulzade78@yahoo.com