RESEARCH ARTICLE
Doi: 10.5578/tt.10207
Tuberk Toraks 2016;64(1):9-16

İyatrojenik imm?n baskılanmış hastalarda pulmoner ?mikrosporidiosisin değerlendirilmesi
Soykan ?ZKO?1, Song?l BAYRAM DELİBAŞ1, ?iler AKIS?1
1 Dokuz Eyl?l ?niversitesi Tıp Fak?ltesi, Tıbbi Parazitoloji Anabilim Dalı, İzmir, T?rkiye
1 Deparment of Medical Parasitology, Faculty of Medicine, Dokuz Eylul University, Izmir, Turkey
?ZET
İyatrojenik imm?n baskılanmış hastalarda pulmoner ?mikrosporidiosisin değerlendirilmesi
Giriş: Microsporidia t?rleri insanın da dahil olduğu geniş bir omurgalı ve omurgasız canlı grubunu infekte edebilen zorunlu h?cre i?i parazitlerdir. Ateş, ?ks?r?k ve dispne gibi nonspesifik semptomlarla karakterize olan pulmoner microsporidiosis, imm?n baskılanmış hastalardaki pulmoner infeksiyonların ayırıcı tanısında ?oğu zaman d?ş?n?lmemektedir. Biz bu ?alışmada, iyatrojenik olarak imm?n baskılanmış hastalardaki pulmoner mikrosporidiyozis prevalansını saptamayı ve hasta karakteristiklerini değerlendirmeyi ama?ladık.
Materyal ve Metod: ?alışma grubuna alınan 63 imm?n baskılanmış hasta ve kontrol grubundaki 28 hastanın BAL sıvısı ?rnekleri, PCR y?ntemiyle incelendi. PCR y?nteminde Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon cuniculi ve Encephalitozoon hellem small-subunit ribosomal DNA (SSU-rDNA) b?lgesine ?zel tasarlanmış PMP1 ve PMP2 primerleri ile 250-279 bp uzunluktaki b?lge amplifiye edildi. Ek olarak PCR pozitif bulunan ?rnekler modifiye trikrom boyası ile Microsporidia spp. sporları y?n?nden incelendi.
Bulgular: İmm?n baskılanmış hastaların 9 (%14.2)'unda Microsporidia spp. PCR pozitifliği saptanırken kontrol grubu hastalarının hi?birisinde pozitiflik g?zlenmedi. İki grup arasındaki bu fark istatistiksel olarak anlamlı bulundu (χ? = 4.439; p= 0.035). Diğer taraftan hastaların yaş ve cinsiyet ?zellikleri ile PCR pozitifliği arasında istatistiksel a?ıdan anlamlı bir ilişki saptanmadı. Mikroskobik değerlendirme sonucunda PCR ile pozitif saptanan dokuz hastanın sadece bir tanesinin BAL ?rneğinde Microsporidia spp. sporları saptandı. Microsporidia spp. sporları saptanmayan sekiz hastadan birinde Mycobacterium tuberculosis, birinde Klebsiella pneumoniae pozitif olarak bulunurken, beş hastanın ?rneğinde Pneumocystis jirovecii DNA'sı pozitifti.
Sonu?: Bu ?alışma, imm?ns?presif hastalardaki pulmoner mikrosiporidiyozisin değerlendirildiği ?lkemizdeki ilk ?alışmadır. ?alışmanın sonu?ları, imm?n baskılanmış hastalardaki pulmoner infeksiyonların ayırıcı tanısında Microsporidia spp.'nin de mutlaka değerlendirilmesi gerektiğini g?stermiş aynı zamanda etkenin laboratuvar tanısında PCR gibi molek?ler y?ntemlerin kullanılmasının ?nemini ortaya koymuştur.
Anahtar kelimeler: Mikrosporidiosis, pulmoner, imm?n baskılanmış
SUMMARY
Evaluation of pulmonary microsporidiosis in iatrogenically immunosuppressed patients
Introduction: Microsporidia spp. are ubiquitous and infect a wide variety of intervertebrates and vertebrates, including humans. Pulmonary microsporidiosis, characterized by nonspecific symptoms like fever, cough and dyspnea, is often overlooked in the differential diagnosis of pulmonary infections in immunsupressed patients. In this study, we aimed to determine the prevalence of pulmonary microsporidiosis in iatrogenically immunosuppressed patients and to evaluate the patient characteristics.
Materials and Methods: Bronchoalveolar lavage (BAL) specimens from 63 iatrogenically immunosuppressed patients and 28 controls were examined with PCR. By using PMP1 and PMP2 common primers specifically designed for Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon cuniculi and Encephalitozoon hellem small-subunit ribosomal DNA (SSU-rDNA) regions at 250-279 bp were amplified. In addition, PCR positive BAL specimens were examined with modified trichrome staining method for Microsporidia spores.
Results: Out of 63 immunosuppressed patients, nine (14.2%) had Microsporidia spp., but none of the control patients had Microsporidia spp. on PCR. This difference between two groups was statistically significant (χ? =4.439; p=0.035). On the other hand there was not a statistically significant relationship between PCR positivity and patient characteristics such as gender and age. Of nine patients with Microsporidia PCR positive, only one had spores of Microsporidia sp. Out of eight patients without spores, one had Mycobacterium tuberculosis, one patient had Klebsiella pneumoniae and five patients had Pneumocystis jirovecii DNA.
Conclusion: This is the first study to evaluate the pulmonary microsporidiosis in immunosupressed patients in Turkey. The results of the study indicated that Microsporidia spp. should be taken into account in the differential diagnosis of pulmonary infections in immunosupressed patients and it is important to use molecular methods such as PCR in the laboratory diagnosis of the causative agent.
Key words: Microsporidiosis, pulmonary, immunosuppressed
Geliş Tarihi/Received: 01.06.2015 • Kabul Ediliş Tarihi/Accepted: 08.10.2015
INTRODUCTION
Microsporidia spp. are obligate intracellular eukaryotic parasites which form small oval spores (1). Although there are about 1200 species of Microsporidia sp. which can infect both vertebrate and nonvertebrated hosts, most of the human microsporidiosis infections are caused by Enterocytozoon sp. and Encephalitozoon spp. (1,2). In recent studies using genetic analyses, the zoonotic potential has clearly been documented about these parasites (3).
The clinical course of microsporidiosis varies with the site of the infection and the host immunity. Asymptomatic or moderate microsporidiosis is likely to remain a local infection in immunocompetent people, while it may present with infections of the hepatobiliary, pulmonary, renal and central nervous systems in addition to severe diarrhea in patients with AIDS and those with iatrogenic immunodeficiency (2,4).
It has been shown that Microsporidia spp. can infect the respiratory tract and the lungs (5). Furthermore, there has an increase in the number of reports on pulmonary microsporidiosis in recent years (6). Pulmonary colonization resulting from contamination through inhalation and regurgitation or oral-fecal and hematogenous routes can lead to opportunistic infections presenting with fever, cough and dyspnea especially in patients with AIDS and transplant recipients (2,5,6,7,8). There have also been reports showing that the parasite can be detected in specimens from the respiratory tract in patients with urinary and intestinal microsporidiosis (6,10). Botterel et al. detected Microsporidia spores in BAL specimens from an AIDS patient with intestinal microsporidiosis and they concluded that the patient had an asymptomatic colonization since he did not have any pulmonary complaints (6).
In Turkey, it has been observed that both clinicians and laboratory specialists do not take account of microsporidiosis in the differential diagnosis of pulmonary infections. Prior studies on this issue mostly focus on revealing intestinal microsporidiosis and a range of 1.05- 69.9% Microsporidia spp. positivities in different patient groups have been reported (11,12,13,14,15,16,17,18,19). However there have not been any studies on the prevalence of pulmonary microsporidiosis in our country. Therefore, in this study we aimed to investigate the pulmonary microsporidiosis in iatrogenically immunosuppressed patients.
MATERIALS and METHODS
Patients
BAL specimens sent to Parasitology Laboratory of Dokuz Eyl?l University Hospital between January 2011 and May 2014 were examined in this study. Sixty-three iatrogenically immunosuppressed patients developing pulmonary infection while being followed in hematology-oncology, bone marrow transplantation, nephrology, rheumatology, chest diseases and intensive care units of the hospital were included into the study group. Twenty-eight immunocompetent patients undergoing bronchoscopy during routine clinical examinations were included into the control group. All patients' records were reviewed and data about underlying diseases, immunosuppressants given, age and gender were collected. Ethical approval was obtained from Non-Invasive Research Ethical Committee of Dokuz Eyl?l University Medical Faculty (number: 2014/23-04).
BAL Specimens and DNA Extraction
BAL specimens were centrifuged at 1500 rpm for 10 min and 200 ?L of obtained pellet was kept at -20?C until DNA extraction. DNA extraction was performed with an extraction kit (Macherey-Nagel, D?ren, Germany) in accordance with the manufacturer's guidelines. Ultrapure distilled water was used as negative control and Enterocytozom cuniculi isolate obtained from ATCC isolate bank (founded in the framework of a project conducted by TUBITAK) was used as positive control.
Microsporidia PCR
Small-subunit ribosomal DNA (SSU-rDNA) regions at 250-279 bp were amplified with the primers PMP1 (5'-CACCAGGTTGATTCTGCCTGAC-3') and PMP2 (5'-CCT CTCCGGAACCAAACCCTG-3') specifically designed for Enterocytozoon bieneusi, Encephalitozoon intestinalis, E. cuniculi and Encephalitozoon hellem (14,18,20,21). For each reaction, 2.5 ?L 10x reaction buffer, 2.5 ?L MgCl2 (25 mM stock solution), 2.5 ?L dNTP (2 mM stock solution),? 1 ?L Hot start Taq DNA polymerase (1 U/?L stock solution), 0.75 ?L primer (10 ?M stock solution) and 5 ?L DNA sample were added and the final volume was completed to 25 ?L with distilled water. Amplification was performed under the following conditions: after an initial denaturation at 94?C for 10 minutes, 35 cycles at 94?C for 30 seconds, at 60?C for 30 seconds, at 72?C for 30 seconds and final extension at 72?C for 10 minutes (20,21). In order to enhance sensitivity, a 5-?L specimen taken from the first amplification product was amplified again with the same primers under the same conditions as in the first reaction (20,21). To evaluate amplification of target DNA, 10 ?L-amplification products were analysed by electrophoresis on 1.5% agarose gel under 100 volt for one hour. Then gel was stained with 1 ?g/mL ethidium bromide and visualized under UV light. PCR was repeated three times for each specimen, for confirmation of results.
Staining
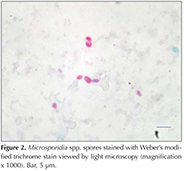
PCR positive BAL specimens were subjected to staining with Weber's modified trichrome after cytocentrifugation and examined under light microscopy at a final magnification of (x) 1000 (14).
Statistical Analysis
Statistical analyses were made with SPSS 15.0. Chi-square test was used for assessing the difference in prevalences. Mann-Whitney U test and Fisher's exact test were used to compare differences between the patients with Microsporidia spp. and those without Microsporidia spp. in the study group. p< 0.05 was considered significant.
RESULTS
In the study group, there were 45 males (71%) and 18 females (29%) with a median age of 58 years (18-71 years). In the control group, there were 19 males (68%) and nine females (32%) with a median age of 63 years (27-66 years). Of 63 patients with iatrogenic immunosuppression, 38 were on anticancer treatment, 14 were on corticosteroid treatment (> 20 mg/day), eight were on anti-rejection treatment (azathioprine, cyclosporine, mycophenolate mofetil etc.) and three were taking other immunosuppressants (methotrexate, TNF-α inhibitors).
Microsporidia PCR positivities were detected in nine out of 63 immunosuppressed patients (14.2%), there was no positivity in immunocompetent patients (Figure 1). The prevalence of Microsporidia spp. was statistically higher in the immunosuppressed patients (χ? = 4.439; p= 0.035).
The median age was higher in PCR positive immunosuppressed patients (median age: 66 years; range: 35-78 years) than in those with PCR negative (median age: 57 years; range: 18-89 years), though the difference was not significant (p= 0.06) (Table 1). There was not a significant difference between PCR positivity and gender (p= 0.255). Characteristics of study and control groups are shown in Table 1.
Cytological investigation of the PCR positive BAL specimens revealed microsporidian spores only in one patient (Figure 2). This patient with multiple myeloma had no other microbial pathogens. Laboratory results from eight patients without microsporidian spores but PCR positivity revealed Mycobacterium tuberculosis in one patient, Klebsiella pneumonia in one patient and Pneumocystis jirovecii in five patients. One patient did not have any other etiological agents. Demographic/clinical characteristics, radiology and laboratory results of the PCR positive patients are shown in Table 2.
DISCUSSION
It is known that Microsporidia spp. cause severe systemic infections in immunosuppressed patients but there is little epidemiological evidence for pulmonary microsporidiosis. It is thought that many possible infections might be overlooked due to not only insufficient focus on microsporidiosis in the differential diagnosis of infections but also difficulties in detection of Microsporidia spp. in laboratory investigations (1,2,3,4).
Most reports about pulmonary microsporidiosis have been stated as case reports in immunosuppressed patients. It is generally observed in patients with AIDS and transplant recipients, may vary from asymptomatic colonization to death from cardiopulmonary failure (7,8,9,10,22,23,24,25,26). On the other hand there has been only one study on the prevalence of pulmonary microsporidiosis. Lobo et al. investigated presence of Microsporidia spp. in BAL specimens from 150 HIV patients and 50 non-HIV patients with PCR in Portugal (27). Although there was no positivity in non-HIV patients, they reported that two HIV patients had Microsporidia spp. So far, only one article has been published in our country about pulmonary microsiporidiasis. Yazar et al. reported that a myeloblastic leukemia (AML-M3) patient presenting with fever, cough, dyspnea and sputum had microsporidian spores in BAL specimens (26). In this study, for the first time, the prevalence of pulmonary microsporidiosis was investigated in Turkey. As far as we know it is the first report which conducted in immunosuppressed patients without HIV. The results showed that 14.2% of the iatrogenically immunosuppressed patients had pulmonary microsporidiosis.
When the literature data about microsporidiosis prevalence analyzed, it was found that researches in our country had mostly dealed with intestinal microsporidiosis. Intestinal microsporidiosis prevalence have been reported between 1.05-69.9 % in patients with different immune status from different geographic regions (11,12,13,14,15,16,17,18,19). The results of these studies show that we often come acroos this zoonotic pathogen. This situation may be one of the reasons of high pulmonary microsporidiosis prevalence that we found in our study.
Pulmonary microsporidiosis has been thought to be due to dissemination of gastrointestinal or urinary tract infections rather than an external transmission (1,2,6). Initiation of pulmonary symptoms following diarrhea due to E. bieneusi in patients with AIDS and isolation of the same causative agent in the patients' pulmonary specimens contributes to the above mentioned view (1,22). This study was directed towards detection of E. cuniculi, E. bieneusi, E. hellem and E. intestinalis, most frequently isolated species in human infections. By using common primers, SSU-rDNA gene of the parasites were amplified. Unfortunately, since genotyping could not be performed to confirm the types of species, all PCR positive specimens were considered as having Microsporidia spp. in general. However since this is a retrospective study, urine and feces specimens from the patients with Microsporidia PCR positivity could not be accessed and could not be evaluated for Microsporidia spp.
The symptoms fever, cough, wheezing, dyspnea and imaging signs such as lobar consolidation are not specific for clinical diagnosis of microsporidiosis (1,5,6,7,8,9,10,22). Researchers had to make their decisions regarding the other pathogens found in BAL specimens. Scaglia et al. reported two AIDS patients having pulmonary symptoms and E. hellem in their BAL specimens. One of these patient was evaluated as Pneumocystis carinii pneumonia (PCP) and the other as tuberculosis. Therefore, the presence of E. hellem was concluded as colonization (9). However, Orenstein et al. incriminated E. cuniculi for fatal pneumonia developing in an acute myeloid leukemia patient undergoing bone marrow transplantation when they could not isolate any other causative agent (8). del Aguila et al. detected Mycobacterium avium complex (MAC) and E. bieneusi in BAL and sputum specimens from a patient with AIDS developing fever and productive cough while they were following the patient for intestinal microsporidiosis. The researchers achieved clinical improvement by offering treatment directed towards the two causative agents (23). The patients in the present study were prone to opportunistic infections due to immunosuppressive therapies. The hospital records of PCR positive patients showed that one patient had M. tuberculosis and another patient had K. pneumoniae. Responses to appropriate antibiotic treatments in these patients suggested that the patients had Microsporidia spp. colonization. Five Microsporidia PCR positive patients were also included in another study about P. jirovecii colonization and nested PCR revealed P. jirovecii DNA (unpublished data). Since they haven't received anti-PCP treatment, their responses to treatment could not be evaluated. It remained unclear whether the patients had PCP or P. jirovecii colonization. As a result, it could not be revealed whether the symptoms were due to microsporidiosis and/or PCP. However, one of the PCR positive patients had Microsporidia spores in BAL specimen. Examination of BAL sediment was negative for P. jirovecii and other microbiological pathogens. He had nonspecific symptoms such as fever, cough and respiratory distress. On physical examination, there were widespread crepitan ralles in both lungs and in radiographic examinations, diffuse, bilateral pulmonary oposities and extensive consolidations areas were determined. The patient did not respond to empirical treatments and died. Pulmonary symptoms in this patient might be caused by Microsporidia spp. The last Microsporidia PCR positive patient who did not have any other microbiological agents in BAL specimen did not receive specific treatments for microsporidiosis. The patient recovered as a result of empirical and supportive treatment and was discharged. Therefore, it was considered as Microsporidia spp. colonization.
CONCLUSION
The present study is the first to investigate the prevalence of pulmonary microsporidiosis in immunosuppressed patients in Turkey.? The results of the study showed that Microsporidia spp. should be taken into consideration in the differential diagnosis of pulmonary infections in iatrogenically immunosuppressed patients and the diagnosis by conventional staining is inadequate and should be supported by molecular methods.
REFERENCES
- Didier ES, Weiss LM. Microsporidiasis: current status. Curr Opin Infect Dis 2006;19:485-92.
- Didier ES, Weiss LM. Microsporidiasis: not just in AIDS patients. Curr Opin Infect Dis 2011;24:490-5.
- Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin Microbiol Rev 2005;18:423-45.
- Sancak B, Aky?n Y. Microsporidia: Genel ?zellikleri, enfeksiyonları ve laboratuvar tanısı. Mikrobiyol Bul 2005;39:513-22.
- Kelkar R, Sastry P, Kulkarni S, Advani S. Pulmonary microsporidial infection in a patient with CML undergoing allogeneic marrow transplantation. Bone Marrow Transplant 1997;19:179-82.
- Botterel F, Minozzi C, Vittecoq D, Bour?e P. Pulmonary localization of Enterocytozoon bieneusi in an AIDS patient: case report and review. J Clin Microbiol 2002;40:4800-1.
- Scaglia M, Sacchi L, Croppo GP, da Silva A, Gatti S, Corona S, et al. Pulmonary Microsporidiasis due to Encephalitozoon hellem in a patient with AIDS. J Infect 1997;34:119-26.
- Orenstein JM, Russo P, Didier ES, Bowers C, Bunin N, Teachey DT. Fatal pulmonary Microsporidiasis due to encephalitozoon cuniculi following allogeneic bone marrow transplantation for acute myelogenous leukemia. Ultrastruct Pathol 2005;29:269-76.
- Scaglia M, Gatti S, Sacchi L, Corona S, Chichino G, Bernuzzi AM, et al. Asymptomatic respiratory tract Microsporidiasis due to Encephalitozoon hellem in three patients with AIDS. Clin Infect Dis 1998;26:174-6.
- Mart?nez-Gir?n R, Esteban JG, Ribas A, Doganci L. Protozoa in respiratory pathology: a review. Eur Respir J 2008;32: 1354-70.
- Atambay M, Karaman U, Daldal N, ?olak C. İn?n? ?niversitesi Turgut ?zal Tıp Merkezi Parazitoloji Laboratuvarına gelen erişkin hastalarda Microsporidium g?r?lme sıklığı. T?rkiye Parazitol Derg 2008;32:113-5.
- Usluca S, Aksoy ?. İmm?n sistemi baskılanmış bir ?ocukta Microsporidium spp. enfeksiyonunun polimeraz zincir reaksiyonu ile tanımlanması. Mikrobiyol Bul 2010;44:679-83.
- Karaman U, Atambay M, Daldal N, ?olak C. Kanser tanısı almış hastalarda Microsporidium g?r?lme sıklığı. Turkiye Parazitol Derg 2008;32:109-12.
- Karaman ?, Daldal N, Atambay M, ?olak C. The epidemiology of Microsporidiasis in humans (Malatya sample). Turk J Med Sci 2009;39:281-8.
- Karaman ?, Şener S, ?alık S, Şaşmaz S. Akut ve kronik ?rtikerli hastalarda Microsporidia pozitiflik oranı. Mikrobiyol Bul 2011;45:168-73.
- T?rk S, Doğruman Al F, Karaman U, Kuştimur S. Investigation of Microsporidia prevalence by different staining methods in cases of diarrhea. Mikrobiyol Bul 2012;46:85-92.
- Turgay N, Unver-Yolasığmaz A, Oyur T, Bardak-?zcem S, T?z S. Monthly distribution of intestinal parasites detected in a part of western Turkey between May 2009-April 2010-results of acid fast and modified trichrome staining methods. Turkiye Parazitol Derg 2012;36:71-4.
- Hamamcı B. Farklı hasta gruplarında Enterocytozoon bieneusi'nin (Protozoa, Microsporıdia) araştırılması. Doktora Tezi, Kayseri: Erciyes ?niversitesi Sağlık Bilimleri Enstit?s?; 2013.
- Hamamci B, ?etinkaya ?, Berk V, Kaynar L, Kuk S, Yazar S. Kemoterapi Alan Kanserli Hastalarda Encephalitozoon intestinalis ve Enterocytozoon bieneusi Prevalansı. Mikrobiyol Bul 2015;49:105-13.
- Samie A, Obi CL, Tzipori S, Weiss LM, Guerrant RL. Microsporidiasis in South Africa: PCR detection in stool samples of HIV-positive and HIV-negative individuals and school children in Vhembe district, Limpopo Province.Trans R Soc Trop Med Hyg 2007;101:547-54.
- Fedorko DP, Nelson NA, Cartwright CP. Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol 1995;33:1739-41.
- Teachey DT, Russo P, Orenstein JM, Didier ES, Bowers C, Bunin N. Pulmonary infection with microsporidia after allogeneic bone marrow transplantation. Bone Marrow Transplant 2004;33:299-302.
- del Aguila C, Lopez-Velez R, Fenoy S, Turrientes C, Cobo J, Navajas R, et al. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal Microsporidiasis. J Clin Microbiol 1997;35:1862-6.
- Hocevar SN, Paddock CD, Spak CW, Rosenblatt R, Diaz-Luna H, Castillo I, et al. Microsporidiasis acquired through solid organ transplantation: a public health investigation. Ann Intern Med 2014;160:213-20.
- Mohindra AR, Lee MW, Visvesvara G, Moura H, Parasuraman R, Leitch GJ, et al. Disseminated microsporidiosis in a renal transplant recipient. Transpl Infect Dis 2002;4:102-7.
- Yazar S, Eser B, Yal?ın Ş, Şahin İ, Ko? AN. A case of pulmonary Microsporidiasis in an acute myeloblastic leukemia (AML)-M3 patient. Yonsei Med J 2003;44:146-9.
- Lobo ML, Xiao L, Antunes F, Matos O. Microsporidia as emerging pathogens and the implication for public health: a 10-year study on HIV-positive and -negative patients. Int J Parasitol 2012;42:197-205.
Yazışma Adresi (Address for Correspondence)
Dr. Soykan ?ZKO?
Dokuz Eyl?l ?niversitesi Tıp Fak?ltesi,
Tıbbi Parazitoloji Anabilim Dalı, İZMİR - TURKEY
e-mail: soykan.ozkoc@deu.edu.tr