RESEARCH ARTICLE
Doi: 10.5578/tt.9973
Tuberk Toraks 2015;63(4):243-249

Plevral t?berk?loz tanısında plevra sıvısı adenozin deaminaz ve
neopterin d?zeylerinin kıyaslanması
Filiz KOŞAR1, Sibel YURT1, Burcu ARPİNAR YİĞİTBAŞ1, Barış ŞEKER1, Hatice KUTBAY ?Z?ELİK2, Hafize UZUN3
1 Yedikule G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği, İstanbul, T?rkiye
1 Clinic of Chest Diseases, Yedikule Chest Diseases and Chest Surgery Training and Research Hospital, İstanbul, Turkey
2 Bezmialem Vakıf ?niversitesi Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, İstanbul, T?rkiye
2 Department of Chest Diseases, Faculty of Medicine, Bezmialem Vakif University, Istanbul, Turkey
3 İstanbul ?niversitesi Cerrahpaşa Tıp Fak?ltesi, Biyokimya Anabilim Dalı, İstanbul, T?rkiye
3 Department of Biochemistry, Faculty of Cerrahpasa Medicine, Istanbul University, Istanbul, Turkey
?ZET
Plevral t?berk?loz tanısında plevra sıvısı adenozin deaminaz ve neopterin d?zeylerinin kıyaslanması
Giriş: Plevral t?berk?loz (PT) tanısında adenozin deaminaz (ADA) ve neopterinin plevral sıvıdaki d?zeylerinin değerini araştırmak ve kıyaslamak.
Hastalar ve Metod: ?alışmaya 50 PT tanılı hasta ile, 27 malign plevral ef?zyon ve 24 nont?berk?loz ve nonmalign plevra sıvısı olan hastalar control grubu olarak dahil edildi. Plevral sıvı ADA ve neopterin d?zeyleri sırasıyla spektrofotometrik ve ELISA y?ntemleri ile tayin edildi.
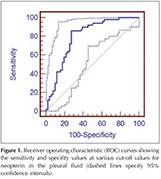
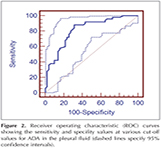
Bulgular: Plevra sıvısında hem? neopterin hem de ADA? d?zeyleri PT'li hastalarda malign hastalardan olduk?a y?ksekti (p< 0.001).? ADA ve neopterinin plevral sıvı ortalama d?zeyleri tanı değerleri altta yatan hastalığa g?re değerlendirildi. Plevral TB i?in ROC eğrisi ile aşağıdaki sonu?lar elde edildi; neopterin i?in en uygun cut-off değer 4.7 U/L olarak hesaplandı ve bu değer i?in sırasıyla sensitivite ve spesifisite of %86 ve %72.55, olarak hesaplandı. ADA i?in cut-off değeri 42 U/L olarak alındığında sensitivite ve spesifisite sırasıyla %88 ve %68.63 olarak hesaplandı.
Sonu?: Plevral TB tanısında plevra sıvısı neopterin d?zeyi ADA aktivitesinin sensitivitesine olduk?a yakın bir tanı değerine sahiptir. Her iki marker de PT?nin erken tanısı i?in rutin değerlendirmede yer alabilirler. Ancak biyopsi ve k?lt?re alternatif olarak d?ş?n?lmemelidirler .
Anahtar kelimeler: Adenozin deaminaz, neopterin, plevra t?berk?lozu, plevral ef?zyon
SUMMARY
The comparative value of pleural fluid adenosine deaminase and neopterin levels in diagnostic utility of pleural tuberculosis
Introduction: The aim of the present study was to evaluate and compare the diagnostic accuracy of pleura levels of adenosine deaminase (ADA) and neopterin for the differential diagnosis of pleural tuberculosis (TP).
Patients and Methods: The study included 50 patients with TB, 27 patients with malignancies, and 24 patients with pleural effusion of non-tuberculous and non-malignant origin as controls. ADA and neopterin levels in pleural fluid were measured by spectrofotometric and ELISA method, respectively.
Results: Pleural neopterin levels were significantly higher in patients with pleural TB than patients with malignancy (p< 0.001).? Pleural ADA levels were significantly higher in patients with pleural TB than patients with malignancy (p< 0.001) and patients with benign non-tuberculosis effusions (p< 0.001). The mean levels of ADA and neopterin in pleural effusion were evaluated according to their underlying diseases for the diagnostic accuracy. As for pleural TB receiving operating characteristic curves identified the following results; The best cut-off value for pleural neopterin was 4.7 U/L and yielded a sensitivity and specificity of 86% and 72.55%, respectively. Taking a cut-off value of 42 U/L for pleural ADA, the sensitivity and the specificity were found to be 88% and 68.63%, respectively.
Conclusions: In the diagnosis of pleural TB pleural neopterin level has a comparable sensitivity to pleural ADA activity. Both markers may find a place as a routine investigation in the coming days for early detection of TB. However, these tests should not be considered an alternative to biopsy and culture.
Key words: Adenosine deaminase, neopterin, pleural tuberculosis, pleural effusion
INTRODUCTION
Pulmonary tuberculosis (TB) remains a public health problem almost worldwide. The diagnosis of TB can be difficult. One third of patients with TB have a negative tuberculin skin test, and only about 5% of these are detected by positive staining for acid-fast bacilli in pleural fluid samples (1). Furthermore, even culture and histological analysis of pleural biopsy samples give negative results in 10-20% of cases (1). In this respect, although it is an infectious disease, various biochemical markers, including cytokines, antibodies and inflammatory markers, have been investigated for several years for the rapid diagnosis of TB (1,2).
Neopterin is a compound derived from pteridine being produced during guanosine triphosphate metabolism. The major source of neopterin is macrophages and monocytes. Macrophages secrete neopterin. Neopterin secretion is a result of the stimulus provided by interferon gamma derived from T lymphocytes and hence it is evaluated as the biological marker of cellular immunity (3,4). Macrophages, but especially T lymphocytes play an important role in tuberculosis immunity. In this sense, studies have been conducted in the evaluation of activation. Increased neopterin levels in various body fluids due to tuberculosis disease activity and immune response were reported (4). While there are studies revealing that neopterin increases in pleural fluid in TB, it is not established to allow routine use (4,5).Adenosine deaminase (ADA) is an enzyme involved in purine catabolism. The enzyme catalyzes the hydrolytic and irreversible deamination of adenosine to inosine and deoxyadenosine to deoxyinosine. ADA has been extensively used in the diagnosis of tuberculous pleural effusion (6).
The aim of the present study was to evaluate and compare the diagnostic accuracy of pleural (p) ADA activity and neopterin levels for the differential diagnosis of TB.
Patients and Methods
Study Design
The study was approved by the Ethics Committee of our hospital (0025-141211-230212-0025) and confirmed written consent forms were obtained from all participants. Clinical and analytic data of 101 consecutive patients admitted to our tertiary center with pleural effusions that were diagnosed by analysis of fluid samples obtained by thoracentesis or chest drainage from March 2012 to April 2013 was prospectively evaluated.
All patients had a history, detailed physical examination, and routine laboratory tests. Flexible bronchoscopy, computed tomography of the chest, and echocardiogram were performed when indicated. Pleural effusions were defined as exudates when the analysis indicated that the fluid satisfied the criteria of Light et al (7). Cytological and microbiological examinations of pleural fluid were also performed.
Pleural effusions were diagnosed as tuberculosis when pleural biopsy findings were positive for pleural tuberculosis and/or when detecting acid-fast bacilli in pleural fluid and Lowenstein-Jensen culture (Tuberculosis Group). Pleural effusions were diagnosed as malignant when fluid cytology or pleural biopsy findings were positive for malignancy or when a primary or metastatic tumour had been diagnosed adjacent to the pleural with no other explanation for the effusion (Malignancy Group). All other exudative effusions were included in the miscellaneous group (Non-tuberculosis non-malignancy Group).
Outcome Parameters
The mean levels of ADA and neopterin in pleural effusionswere evaluated according to their underlying diseases for the diagnostic accuracy. ADA activity in the pleural fluid was studied with De Giusti method and the results were recorded as IU/L in all the patients (8). A pleural sample was centrifuged at 3000 x g for 10 min. within half an hour and stored at -20?C until assay.
Assay of Pleural Effusion ADA Activity
The manual kinetic pleural effusion ADA activity assay was optimized for the automated analyzer (Konelab 60 I). For the determination of ADA activity, the ammonia produced by the enzymatic activity was coupled to 2-oxoglutarate by glutamate dehydrogenase. 2-oxoglutarate was activated by adenosine diphosphate (ADP). In this reaction, NADH was used as indicator and the reaction was followed by the decrease of absorbance at 340 nm. This method was developed by Ellis (9). All chemicals for ADA assay were obtained from Sigma. Intra and inter-assay coefficients of variation for ADA were 8.7% and 9.8%, respectively.
Assay of Pleural Effusion Neopterin Concentrations
Pleural effusion neopterin levels were determined by ELISA (DRG Instruments Gmbh, Germany, EIA-1476) in accordance with the recommendations of the manufacturers. Intral and interassay coefficients of variation for neopterin were 9.1% and 9.9%, respectively.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) software version 21.0 (SPSS Inc., Chicago, IL) and Medcalc 9 (Mariakerke, Belgium) for Windows. A normal distribution of the quantitative data was checked using Kolmogorov-Smirnov and Shapiro-Wilk tests. Parametric tests were applied to data of normal distribution and non-parametric tests were applied to data of questionably normal distribution. Independent-samples t-test was used to compare independent groups; while, paired-samples T test was used to compare dependent groups. Repeated Anova test was used for multiple comparisons. Scheffe and Games Howel tests were used for post-hoc comparisons. The distribution of categorical variables in both groups was compared using Pearson chi-square test. Receiver operating characteristic (ROC) curves were used to identify the optimal cut-off points. Data are expressed as mean ? SD (standard deviation). Categorical variables are expressed as frequencies and percentages. Statistical significance was assumed for p< 0.05.
RESULTS
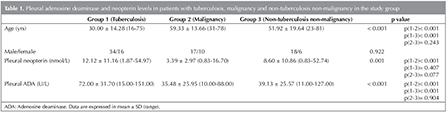
The study population included 69 men and 32 women. According to clinical diagnosis, the study population (101 patients) was distributed into the three groups as follows: 50 patients (34 males, 16 females) with pleural TB confirmed by the detection of Mycobacterium tuberculosis in pleural fluid, or pleural biopsy specimens, either by microscopy and/or culture (5 patients), or the histological demonstration of caseating granulomas in the pleura along with acid fast bacilli (AFB) (45 patients), 27 patients (17 males, 10 females) with malignancy, and 24 patients (18 males, 6 females) with benign nontuberculous effusion (pleural effusion due to an etiology other than TB or malignancy). Within the group with nontuberculous/nonmalignant effusion, 3 patients had effusions associated with asbest, 1 with renal insufficiency, 17 with bacterial pneumonia or nonspecific pleural effusion may be viral and 1 with romatoid arthritis, 1 with pulmonary embolism, 1 with systemic lupus eritematosus. There were no statistically significant difference for gender among groups (p= 0.922).
The mean age was 30.00 ? 14.28 (range 16 to 75) years for the Tuberculosis Group, 59.33 ? 13.66 (range 31 to 78) years for the Malignancy Group, and 51.92 ? 19.64 (range 23 to 81) years for the Nontuberculosis nonmalignancy Group. There were statistically significant difference for age between Tuberculosis Group and other groups (p< 0.001 for each comparison); whereas no significant difference was observed between Malignancy Group and Nontuberculosisnonmalignancy Group (p= 0.243).
Comparative characteristics pleural neopterinand pleural ADA levels among patients with tuberculosis, patients with malignancy and patients with benign non-tuberculosis effusions presented in Table 1. Pleural neopterin levels were significantly higher in patients with pleural TB than patients with malignancy (p< 0.001). There were no significant differences in neopterin levels between patients with pleural TB and patients with nontuberculosis/nonmalignant effusions (p= 0.407). Also, there were no significant differences in neopterin levels between patients with malignancy and patients with nontuberculosis/nonmalignant effusions (p= 0.077). Pleural ADA levels were significantly higher in patients with pleural TB than patients with malignancy (p< 0.001) and patients with nontuberculosis/nonmalignant effusions (p< 0.001). There were no significant differences in ADA levels between patients with malignancy and patients with nontuberculosis/nonmalignant effusions (p= 0.904).
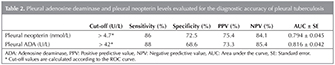
As for pleural TB receiving operating characteristic (ROC) curves identified the following results (Table 2) (Figure 1,2). The best cut-off value for pleural neopterinwas 4.7 U/L and yielded a sensitivity and specificity of 86% and 72.55%, respectively. Taking a cut-off value of 42 for pADA, the sensitivity and the specificity were found to be 88% and 68.63%, respectively. For pleural TB cases, the area under the curve (AUC) of the ROC curve was higher for pleural ADA (0.816 ? 0.042) then pleural neopterin (0.794 ? 0.045) (Table 2).
DISCUSSION
In this study, we aimed to evaluate and compare the effectiveness of pleural neopterin and ADA activity for the differential diagnosis of pleural TB. Our study showed that the quantification of neopterin and the measurement of ADA have shown high sensitivity and specificity in pleural TB.
It is well known that because of the low number of M. tuberculosis in pleural effusion, sensitivity of various methods of M. tuberculosis identification in pleural fluid is low (10). The highest diagnostic sensitivity, reaching 70-80%, has been observed for PCR methods identifying specific nucleic acid sequences in pleural fluid. These diagnostic methods are, however, associated with a relatively high rate of false positive results (11). The search for effective but less invasive methods to accurately diagnose tuberculous pleural effusion has been going on for years. Several biological markers measured in pleural fluid have been found to be sensitive and relatively specific indices of tuberculous pleurisy. These markers include: ADA, IFN-, neopterin and other cytokines or substances detectable in pleural effusion (12).
In the past decade a large number of biochemical parameters (e.g., IL-6, IL-12, IL-18, soluble IL-2 receptor, TNF-) have been evaluated as diagnostic tools for pleural effusions. In the case of TB, one of the most explored is ADA, which is a polymorphic enzyme that catalyzes the deamination of adenosine to inosine and ammonia (6). In numerous studies ADA has proven to be an accurate and valuable test for differential diagnosis of TB (12,13,14,15,16,17). Elevated ADA activity is not specific for TB, but is also seenin parapneumonic effusions and pleural empyemas, rheumatoid effusions, and certain malignant pleural effusions. The present study revealed high level of ADA in pleural fluid of biopsy proven cases of tuberculous pleural effusion as compared to non-tuberculous group. Chen et al. revealed a cut-off value of ADA for TB was 55.8 U/L (specificity= 91.8%, sensitivity= 87.3%, PPV= 82.1%, NPV= 94.4%) (12). In the study published by Valdes et al., the cut-off value for ADA was 47 U/L, sensitivity was 100%, and specificity was 91 %(13). Diaconet al. found that the cut-off value for TB was 50 U/L, sensitivity was 95%, and specificity was 89% (2). A study conducted by Gorguner et al. also revealed results similar to ours, i.e. cut-off value= 47 U/L, sensitivity= 91%, specificity= 89%, and PPV= 82% (14). The higher specificity and sensitivity may be explained by higher tuberculosis incidence in the study region. Salazar-Lezama et al. found that ADA is as good as pleural biopsy in the diagnosis of TB(15). Burgess et al. found sensitivity and specificity of ADA to be 90% and 89%, respectively, at the cut-off value of 50 U/L (16). In the present study, taking a cut-off value of 42 U/L for pleural ADA, the sensitivity and the specificity were found to be 88% and 68.63%, respectively. Sensitivity level is consistent with the values in literature, low spesifity can be explained with relatively low cut-off value (42 U/L).
Neopterin is produced by activated macrophages in response to interferon gamma of lymphocyte origin. It was reported that neopterin increases in various body fluids in patients with tuberculosis where cellular immunity plays an important role (17,18,19,20). In our study, pleural fluid neopterin levels in the TB group were significantly higher than the malignancy group. Baganha et al. was the first to compare 10 TB patients and 15 patients with malignancy and reported that neopterin levels were higher in the pleural fluid in TB group and thus it can be used in determining the pleural fluid aetiologyand cellular immunity (17). Chiang et al. reported that pleural neopterin is lower in patients with malignant pleurisy than it is in patients with TB and that pleural neopterin level is below 25 nmol/L in 84% of the patients with malignancy (18). Similarly, the neopterin level was found to be below 25 nmol/L in all of the patients with malignancy, in our study. Tozkoparan et al. studied 34 patients with TB and 29 patients with malignancy, parapneumonic effusion, congestive heart failure, empyema, pulmonary thromboembolism and liver cirrhosis, for the purpose of determining the cut-off point (4).? They decided on 30 nmol/L as the cut-offvalue and found the sensitivity as 85%, specificity as 93%, PPV as 94% and NPV as 84% (4). As for pleural TB, ROC curves identified that the best cut-off value for pleural neopterin was 4.7 U/L and yielded a sensitivity and specificity of 86% and 72.55%, respectively. For pleural TB cases, the area under the curve of the ROC curve was 0.794 ? 0.045. ?ok et al. reported that although neoptrin levels of pleural TB were higher than malignant effusions, its clinical use as a diagnostic marker was not valuable as ADA in their study which compare the diagnostic values of ADA and neopterin in 16 TB and 19 malignant? effusions (5). Our study has the greatest number of patients comparing TB, malignant and nontuberculous/nonmalignant effusion in distinguishing pleural TB, pleural ADA activity has higher sensitivity than pleural level of neopterin. As pleural levels of ADA in tuberculous pleurisy were significantly higher than in malignant and also in nonmalignant-nontuberculous pleural effusions, pleural levels of neopterin in TB? were only significantly higher than malignant pleural effusions.
It must be kept in mind that serological tests provide unpredictable findings resulting in exceedingly variable values for sensitivity and specificity. There is no evidence that existing commercial serological assays improve patient-important outcomes, and high proportions of false-positive and false-negative results adversely impact patient safety (7). Cost and expertise are also considerations in adopting technologies for use at different levels of health service. Neopterin and ADA measurements are simple and comparatively low-cost procedures, where as PCR is a more demanding and expensive method yet has the advantage of being a rapid and direct means of detecting M. tuberculosis in pleural fluid. We believe that the results of this study demonstrate that individually and in combination, these methods can offer a cost-effective means of obtaining diagnostic efficiency. The results should be interpreted with caution. However, we hope that this study will pioneer further studies for the non-invasive differential diagnosis of pleural TB.
In conclusion, in the diagnosis of pleural TB pleural neopterin level has a comparable sensitivity to pleural ADA activity. Both markers may find a place as a routine investigation in the coming days for early detection of TB. Nevertheless, these tests should not be considered an alternative to biopsy and culture. Further randomized, prospective, controlled trials on larger series are necessary for making more precise interpretations. Cultures of pleural fluid and biopsy specimens have a greater diagnostic yield.
REFERENCES
- Valdes L, Pose A, San Jose E, Mart?nez Vazquez JM. Tuberculous pleural effusions. Eur J Intern Med 2003;14:77-88.
- Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91.
- Anwaar I, Gottsa E'ter A, Hedblad B, Palmqvist B, Mattiasson I, Lindga E'rde F. Endothelial derived vasoactive factors and leukocyte derived inflammatory mediators in subjects with asymptomatic atherosclerosis. Angiology 1998;49:957-66.
- Tozkoparan E, Deniz O, Cakir E, Yaman H, Ciftci F, Gumus S, et al. The diagnostic values of serum, pleural fluid and urine neopterin measurements in tuberculous pleurisy. Int J Tuberc Lung Dis 2005;9:1040-5.
- Cok G, Parildar Z, Basol G, Kabaroglu C, Bayindir U, Habif S, et al. Pleural fluid neopterin levels in tuberculous pleurisy. Clin Biochem 2007;40:876-80.
- Lima DM, Colares JK, da Fonseca BA. Combined use of the polymerase chain reaction and detection of adenosine deaminase activity on pleural fluid improves the rate of diagnosis of pleural tuberculosis. Chest 2003;124:909-14.
- Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13.
- Giusti G, Galanti B. Methods of enzymatic analysis. Verlag Chemie Weinheim 1984;315.
- Ellis G, Goldberg DM. A reduced nicotinamide adenine dinucleotide linked kinetic assay for adenosine deaminase activity. J Lab Clin Med 1970;76:507-17.
- Li L, Qiao D, Fu X, Lao S, Zhang X, Wu C. Identification of Mycobacterium tuberculosis-specific Th1, Th17 and Th22 cells using the expression of CD40L in tuberculous pleurisy. PLoS One 2011;6:e20165.
- Tang TH, Ahmed SA, Musa M, Zainuddin ZF. Rapid detection of Mycobacterium tuberculosis in clinical samples by multiplex polymerase chain reaction (mPCR). World J Microbiol Biotechnol 2013;29:2389-95.
- Chen ML, Yu WC, Lam CW, Au KM, Kong FY, Wo Cham AY. Diagnostic value of pleural fluid adenosine deaminase activity in tuberculous pleurisy. Clin Chim Acta 2004;341:101-7.
- Valdes L, San Jose E, Alvarez D, Valle JM. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 1996;9:747-51.
- Gorguner M, Cerci M, Gorguner I. Determination of adenosine deaminase activity and its isoenzymes for diagnosis of pleural effusions. Respirology 2000;5:321-4.
- Slazar-Lezama M, Torrentera RG, Bouscoulet L. Comparison of adenosine deaminase levels in pleural fluid versus pleural biopsy, culture and smear in 124 consecutive patients with tuberculous pleural effusions. Chest 2002;122:90S.
- Burgess L, Martiz F, Le Roux I, Taljaard JJF. Use of adenosine deaminase as a diagnostic tool for tuberculous pleurisy. Thorax 1995;50:672-4.
- Baganha MF, Mota Pinto A, Pego MA, Marques MA, Rosa MA, Cordeiro AJ. Neopterin in tuberculous and neoplastic pleural fluids. Lung 1992;170:155-61.
- Chiang CS, Chiang CD, Lin JW, Huang PL, Chu JJ. Neopterin, soluble interleukin-2 receptor and adenosine deaminase levels in pleural effusions. Respiration 1994;61:150-4.
- Mohamed KH, Mobasher AA, Yousef AR, Salah A, El-Naggar IZ, Ghoneim AH, et al. BAL neopterin: a novel marker for cell mediated immunity in patients with pulmonary tuberculosis and lung cancer. Chest 2001;119:776-80.
- Yuksekol I, Ozkan M, Akgul O, Tozkoparan E, Al-Rashed M, Balkan A, et al. Urinary neopterin measurement as a noninvasive diagnostic method in pulmonary tuberculosis. Int J Tuberc Lung Dis 2003;7:771-6.
Yazışma Adresi (Address for Correspondence)
Dr. Filiz KOŞAR
Yedikule G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi
Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği,
İSTANBUL - TURKEY
e-mail: filizkosar@gmail.com