RESEARCH ARTICLE
Doi: 10.5578/tt.9753
Tuberk Toraks 2015;63(3):158-164

YKL-40: Obstr?ktif uyku apne sendromunda yeni bir inflamatuvar biyobelirte? olarak kullanılabilir mi?
Serap DURU1, G?lbahar Y?CE1, Hikmet FIRAT1, Beng?l ŞİMŞEK1, Fatma U?AR2, Sadık ARDI?3,
Emine Bahar KURT1
1 Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği, Ankara, T?rkiye
1 Clinic of Chest Diseases, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey
2 Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi, Klinik Biyokimya Laboratuvarı, Ankara, T?rkiye
2 Clinical Biochemistry Laboratory, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey
3 Acıbadem Hastanesi, G?ğ?s Hastalıkları Kliniği, Ankara, T?rkiye
3 Clinic of Chest Diseases, Acibadem Hospital, Ankara, Turkey
?ZET
YKL-40: Obstr?ktif uyku apne sendromunda yeni bir inflamatuvar biyobelirte? olarak kullanılabilir mi?
Giriş: YKL-40 [chitinase-3 like-1 (CHI3L1)], inflamasyon, endotel disfonksiyonu, remodellingde rol oynayan ve inflamasyon i?in noninvaziv prognostik biyobelirte? olarak kabul edilen bir glikoproteindir. Bu ?alışmada, obstr?ktif uyku apne sendromlu (OSAS) hastalarda YKL-40'ın bir inflammatory biyobelirte? olarak kullanılabilirliğini g?stermeyi ama?ladık.
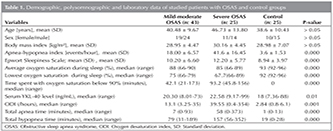
Hastalar ve Metod: ?alışmaya iki grup OSAS'lı hasta [Grup I: Hafif-Orta OSAS; n: 43, apnea-hypopnea indeksi; AHI, /saat: 18 (7.1-28.2), Grup II: Ağır OSAS; n: 25, AHI: 41.6 (33.9-86.9)] ve sağlıklı kontrol grubu [n: 25, AHI: 3.6 (0.4-5) ] alındı. Polisomnografi sonrası OSAS'lı hastalar ve kontrol grubunun serum YKL-40 d?zeyleri incelendi. Ayrıca OSAS'lı hasta gruplarında serum YKL-40 d?zeyinin yaş, v?cut kitle indeksi ve polisomnografik parametreler ile olan ilişkisi araştırıldı.
Bulgular: Ortalama serum YKL-40 d?zeyi hafif-orta OSAS'lı hastalarda 20.30 ng/mL (8.01-73 ng/mL), ağır OSAS'lı hastalarda 22.58 ng/mL (9.17-99 ng/mL) ve kontrol grubunda 18 ng/mL (7.36-88 ng/mL) olarak bulundu (p= 0.01). Hafif-orta OSAS'da AHI ile (p< 0.05), ağır OSAS'ta ise yaş, total hipopne s?resi (dakika) ile serum YKL-40 d?zeyi arasında (p< 0.05) korelasyon olduğu g?r?ld?. Ancak diğer değişkenler ile serum YKL-40 d?zeyi arasında ilişki saptanmadı (p> 0.05).
Sonu?: Bu ?alışma sonucunda OSAS'ın ağırlığı ile serum YKL-40 d?zeyinin birlikte arttığını saptadık. Bu bulgular YKL-40'ın OSAS'lı hastalarda inflamasyon i?in kullanılabilir bir biyobelirte? olabileceğini d?ş?nd?rmektedir.
Anahtar kelimeler: YKL-40, belirte?, obstr?ktif uyku apne sendromu
SUMMARY
YKL-40: may be use as a new inflammatory biomarker in obstructive sleep apnea syndrome?
Introduction: YKL-40 [chitinase-3 like-1 (CHI3L1)] is a glycoprotein, has been implicated in inflammation, endothelial dysfunction, tissue remodelling and it is accepted as a noninvasive prognostic biomarker for inflammation. In this study, we aimed to underline usability of serum YKL-40 as an inflammatory biomarker in patients with obstructive sleep apnea syndrome (OSAS).
Patients and Methods: Two groups OSAS patients [Group I: Mild-moderate OSAS, n:43; median apnea-hypopnea index: AHI, /hour:18], Group II: Severe OSAS, n: 25; AHI:41.6] and healthy control group [n:25, AHI: 3.6] were included in the study. Serum YKL-40 level was tested in serum samples taken after polysomnography in OSAS patients and control group. In addition, the association of serum YKL-40 level with age, body mass index and polysomnografic parameters were analyzed in the OSAS patient groups.
Results: Median serum YKL-40 level was 20.30 ng/mL (range 8.01-73 ng/mL) in mild-moderate OSAS patients, and 22.58 ng/mL (9.17-99 ng/mL in severe OSAS patients, 18 ng/mL (range 7.36-88 ng/mL) in control group (p< 0.05). Serum YKL-40 level was found to be correlated with AHI in patient with mild-moderate OSAS patients (p< 0.05) and serum YKL-40 level was found to be correlated with age, total hypopnea time (minutes) in severe OSAS patients (p< 0.05). There was no relationship serum YKL-40 level with other studied variables (p> 0.05).
Conclusion: At the end of this study, we found that serum YKL-40 level increase with severity of OSAS. The findings suggest that YKL-40 may be a useful biomarker for inflammation in patients with OSAS.
Key words: YKL-40, marker, obstructive sleep apnea syndrome
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a syndrome characterized by respiratory standstill episodes during sleep, oxygen desaturation, and excessive sleepiness over the day caused by repetitive obstructions of upper airways (1,2). The mean frequency in adults is between 2-4% (3). Numerous risk faktors such as obesity, advanced age, race, anatomical disorders in the head and neck, male gender, smoking, alcohol and sedative drugs, high altitude play a role in the development of OSAS, the golden standard for diagnosis is polysomnography (PSG) (4,5). Apnea and hypopnea defined by PSG datas (6). Apnea-hypopnea index (AHI, events/hour) is used to determine the severity of the disease (7,8).
In recent years, increasing number of studies are available on OSAS, of which the pathopysiology is not fully explained yet, which are related to the inflammatory process, with particular emphasis on cytokine and chemokine responses (9,10). Moreover, the presence of local and systemic inflammation in OSAS was demostrated, and it was suggested that this plays a role in the clinical picture (11). C reactive protein (CRP), leptin, TNF alpha, interleukin-6, reactive oxygen radicals and adhesion molecules are the most powerful proinflammatory markers known in the systemic inflammation in OSAS.
YKL-40 [human cartilage glycoprotein-39 (HC-gp39), chitinase-3 like-1 (CHI3L1)]? is a heparin- and chitin-binding glycoprotein. YKL-40, of which the physiological functions are not precisely known, has no chitinase activity (12,13,14,15). YKL-40 has angiogenetic effect in vascular endothelium cells, and plays role in inflammation, remodelling (16). Previous studies have shown, that the expression of YKL-40 is increased by the inflammatory action of T hepler type 2 cells (17). In some disorders of the central nervous system, serum YKL-40 level is altered. While in neuroinflammatory disorders, YKL-40 expression from astrocytes is seen, in brain tumors, in traumatic brain injury, and in Alzheimer disease, an increase of serum and tissue YKL-40 levels was established (18,19,20,21,22). Our aim in this study is to evaluate the relationship of serum YKL-40 level with the severety of the disease in OSAS where the inflammatory system is affected, and to show its importance as a biomarker.
Materials and Methods
Ethics Committee Approval
Written consent form was obtained from all patients in the study. Furthermore, the study was approved by the ethics committee of the Dışkapı Yıldırım Beyazıt Training and Reseach Hospital.
Patients
To the study, 93 (53 male) patients were enrolled, who have been followed up in Dışkapı Yıldırım Beyazıt Training and Reseach Hospital Sleep Laboratory between June-December 2012.
The demographic data of the patient, their smoking and alcohol drinking habits, as well as, their additional disorders were examined. As exclusion criteria, acute and chronic infections in the last three months, myocardial injury, history of cancer, restless leg syndrome, anatomical nose, neck and jaw problems, sleep phase disorders, use of alcohol and drugs affecting the sleep phase (such as hypnotics), chronic airways disorders, take anti-inflammatory drugs in the last month, inflammatory disorders, diabetes were adopted.
Method
Detailed general complaints and especially their sleep problems were recorded. Detailed physicals exams, detailed blood tests, height, weight and neck circumference measurements were conducted. The body mass index (BMI, kg/m2) of each patient was calculated by dividing the body weight in kilograms by the square of the patient's height in meters. Assuming its benefit in exclusion of the respiratory system pathologies, posteroanterior chest radiography was performed. Pulmonary function tests were performed in the respiratory laboratory of our hospital, using a Jaeger brand spirometer according to the criteria of American Thoracic Society (ATS). Forced expiratory volume in one second (FEV1, liters), and forced vital capacity (FVC, liters), and FEV1/FVC (%) values are measured at least 3 times, and optimal values were recorded.
Polysomnography
Patients with common complaints of snoring, witnessed apnea and/or daytime excessive sleepiness, were first given an endoscopic examination by otorhinolarnygologist. Subjects with anatomical nasal, neck and jaw problems (septal deviation, concha hypertropy, retro-micrognathia, etc.) were excluded from the study. On the day of the polysomnographic tests of patients with OSAS, previously validated Turkish version of the Epworth sleepeness scala (23). To patients, full-night polysom -nographic tests with 64-channel Compumedics brand E-Series Polysomnography apparatus were applied (for 6-8 hours). 6-channel electroencephalogram (EEG) (C3-M2, C4-M1,F1-M2 O1-M2, O2-M1,F2-M1), electroloculogram (EOG), electromyogram (submental EMG and EMG Tibialis), electrocardiogram (EKG), oronasal airflow (cannula + thermistor), respiratory effort (chest and abdomen), snoring (tracheal microphone), body position (body position), pulse oxymetry (fingertip) parameters were evaluated with Profusion version 3 software program.
AASM-2007 (American Academy of Sleep Medicine) criteria were used for manual scoring of sleep phase and respiratory events (apnea, hypopnea, desaturation, etc.). Tests with sleep effectiveness values of minimum 60%? were evaluated as valid. Apnea was evaluated as respiratory standstill for more than 10 second, time must provide ≥ 90% amplitude criteria, in air flow signal measured by thermal sensor must decreases ≥? 90%, hypopnea defined as a > or = 30% reduction in airflow and thoracoabdominal excursion both of which are accompanied by a > or= 4% drop in oxyhemoglobin saturation.
As polysomnography parameters, AHI, average oxygen saturation during sleep (%), lowest oxygen saturation after apnea (%), and time with oxygen saturation below 90%, hourly desaturation events of equal or greater than 4% (Oxygen desaturation index, ODI, /hours), total apnea time (minutes), total hypopnea time (minutes), average apnea time (minutes), average hypopnea time (minutes) were recorded. Following morning PSG, fasting venous blood samples were collected. Serum samples collected for YKL-40 (ng/mL) were centrifuged at 3000 rotations for 5 minutes and stored at -70?C.
Human YKL-40 enzyme-linked immunosorbent assay (ELISA) kit (Adipobiotech, 20110516, Santa Clara, USA) was used to measure the serum YKL-40 level.
After completing overnight PSG, we classified subjects either as controls with an Apnoea- Hypopnoea Index (AHI) < 5 events/h or as OSAS patients with an AHI ≥ 5 events/h. Patients were divided into three groups based on AHI, and analysis was performed within each group as follows: normal (AHI < 5; n= 25), mild-moderate OSAS (AHI between 5 and 30; n= 43),? severe OSAS (AHI ≥ 30; n= 25). There was found 34 patients in AHI between? 5-15 events/h and 32 patients in AHI> 15 events/h.
The relationship between measured serum YKL-40 level and the severity of OSAS in the patients in the study and PSG parameters was investigated.
Statistical Method
SPSS 15.0 (SPSS Inc; Chicago, III) package program was used for statistical analysis of the data. As the measure of the average, mean ? SD, or, when normal distribution was not the case, median (min-max) was used. Regarding the variables exhibiting normal distribution, in order to investigate whether there is any difference between the AHI ≤ 5, AHI: 5-30 and AHI ≥ 30 and higher groups, one-way analysis of variance was performed for those meeting the homogeneity of the variances requirement. When P< 0.05, it was told that there was a difference between the groups. Then, Least Square Deviation (LSD) was performed to determine the group that was different. For those not meeting the homogeneity of the variances requirement, Welch test was performed. Dunnett T3 test was performed to determine the group that was different. For the variables that do not fulfill the requirement of the normality, Kruskal-Wallis H test was performed. Different groups were determined by Mann-Whitney U test and Bonferroni correction.
In order to correlation between the variables, Pearson correlation coefficient was used for the variables exhibiting a normal distribution, and Spearman correlation coefficient was used for the variables not exhibiting a normal distribution. Correlations with P< 0.05 were interpreted as significant.
RESULTS
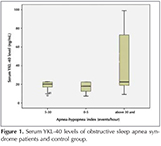
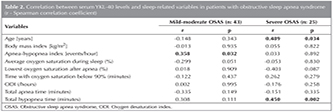
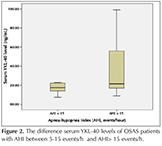
Demographic and PSG data of the patient and control grups included in the study were shown in Table 1. Serum YKL-40 level, was found to be significantly higher in the severe OSAS group than other groups (p< 0.05). In Figure 1, patients' serum YKL-40 levels were shown. The relation between serum YKL-40 level and other variables was shown in Table 2. In the severe OSAS group, a positive correlation was seen with the age, total hypopnea time and in the mild-moderate OSAS group, with the AHI and serum YKL level (p< 0.05). No correlation was found between serum YKL-40 level and BMI and other PSG parameters. There was no gender difference? for serum YKL-40 level in all groups with OSAS (p> 0.05). The difference (p> 0.012) was found patients with AHI between 5-15 events/h (n= 37) and patients with AHI > 15 events/h (n= 31), (Figure 2).
DISCUSSION
In this study, it was determined that in severe OSAS patients, serum YKL-40 level was increased compared to the mild-moderate OSAS and control groups. Furthermore, it was seen that, there was a positive correlation with AHI in mild-moderate OSAS. Also there were a positive correlation age, total hypopne time and serum YKL-40 level in severe OSAS. However, no relationship between other PSG parameters and serum YKL-40 level was established.
In recent years, it was shown that serum YKL-40 level is increased in inflammation, endothelial dysfunction, angiogenesis and remodelling (24,25). As a result of the increase of serum YKL-40 level in many disorders such as asthma, diabetes, rheumatoid arthritis, malignancies, hepatic fibrosis, is being proposed that it may be used as a non-invasive prognostik biomarker (26,27,28,29,30).
There is a limited number of studies on serum YKL-40 level and related neurological disorders. The number of the studies regarding the YKL-40 expression in the neurological system, and especially in brain tumors is gradually increasing. The fact that in previous studies, YKL-40 expression by microglia and astrocytes was shown, may suggest an increase of YKL-40 expression in OSAS as well (19,31).
Moreover, according to our results, since the increase in serum YKL-40 level severe OSAS patients is higher than those in mild-moderate OSAS patients and control group, it might suggest that the severity of the disease and the increase in serum YKL-40 level are correlated. In the literature, it was demonstrated that the expression YKL-40 is modulated by the proinflammatory cytokines IL-1, TNF-α, and IL-6 from chondrrocytes, macrophages and glioblastoma cells, and it was told that YKL-40 may play a role in tissue remodelling in inflammation (32,33,34). Also, in OSAS, inflammatory cytokines are known to increase. The increase of YKL-40 expression in acute and chronic inflammatory neurological disorders (such as encephalitis, meningitis, multiple sclerosis, ALS), may suggest that in OSAS, the expression YKL-40 by astrocytes and glial cells might take place (19). Furthermore, in our study, higher serum YKL-40 levels measured after PSG found in OSAS patients than in control group, are likely to suggest serum an increase of YKL-40 levels as a result of the inflammation induced by the hypoxia overnight.
Is it proposed that advanced age is a risk factor for OSAS (35). Additionally, in previous studies, it was demonstrated that the increase in AHI level is correlated with the inflammation in OSAS (36). The positive correlation of AHI level and serum YKL-40 level only for? mild-moderate OSAS suggests that it may be a biomarker useful in the evaluation of OSAS.
In our study, the average age in the severe OSAS group was found to be higher than that in the other groups. In the severe OSAS patient group, the positive increase of the age and serum YKL-40 level might suggest an increases of inflammation with age in OSAS. Additionally, in our severe OSAS patient group, the determination of the correlation between total hypopnea time and serum YKL-40 level, supports the idea that YKL-40 can be used as a biomarker in OSAS. Continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BPAP) are the gold standard treatments for obstructive sleep apnea syndrome (OSAS). The OSAS patients with AHI > 15 events/hour is effectively treated with CPAP. In our study shown that serum YKL-40 is high level in OSAS patients with AHI > 15 events /hour.
Serum YKL-40? level may be useful biomarker during OSAS follow up and in evaluating the efficacy of the treatment. There is a need for multicenter studies with a large number of patients.
When we look at the literature, we could not come across with a study measuring serum YKL-40 level in OSAS. In our study, serum YKL-40 level was evaluated in OSAS patients without an additional inflammatory disorder. Therefore, our results suggest that the increase in serum YKL-40 level might be related to the inflammation in OSAS, and that there is a need for conducting new research on this subject.
Limitations of the Study
Although the number of registered follow-up patients in our clinic is high, them having additional disorders restricted our number of patients. The results of studies conducted with more patients may differ.
Conclusion
In OSAS, a disorder that gained importance due to its prevalance in the population, might suggest that the increased serum YKL-40 level with the severity of the disease may be used as an inflammatory biomarker.
Conflict of interest
None declared.
REFERENCES
- Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstruktive sleep apnea syndrome patients: mortality. Chest 1988;94:1200-4.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5.
- Demir A, Ursavaş A, Tana Aslan A, G?lbay B, ?ift?i B, ?uhadaroğlu ?, et al. Obstr?ktif uyku apne sendromu tanı ve tedavi uzlaşı raporu. T?rk Toraks Derneği 2012;1-73.
- Thropy MJ, Broughton RJ, Cohn MA, et al. American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual (ICSD-2). 2nd ed. American Academy of Sleep Medicine: Westchester, IL, 2005.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF, American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine: Westchester, IL, 2007.
- Meoli AL, Casey KR, Clark RW, Coleman JA Jr, Fayle RW, Troell RJ, et al. Hypopnea in sleep-disordered breathing in adults. Sleep 2001;24:469-70.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci 2009;10:199-210.
- Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, et al. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006;166:1725-31.
- Arter JL, Chi DS, Girish M, Fitzgerald SM, Guha B, Krishnaswamy G, et al. Obstructive sleep apnea, inflammation and cardiopulmonary disease. Front Biosci 2004;1:2892-900.
- Johansen JS, Williamson MK, Rice JS, Price PA. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res 1992;7:501-12.
- Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 1993;268:25803-10.
- Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem 2006;281:21082-95.
- Funkhouser JD, Aronson NN Jr. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 2007;7:96.
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 2006;53:172-209.
- Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 2009;206:1149-66.
- Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol 2012;22:530-46.
- Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation 2010;7:34.
- Zhang W, Kawanishi M, Miyake K, Kagawa M, Kawai N, Murao K, et al. Association between YKL-40 and adult primary astrocytoma. Cancer 2010;116:2688-97.
- Bonneh-Barkay D, Zagadailov P, Zou H, Niyonkuru C, Figley M, Starkey A, et al. YKL-40 expression in traumatic brain injury: an initial analysis. J Neurotrauma 2010;27:1215-23.
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry 2010;68:903-12.
- Izci B, Ardic S, Firat H, Sahin A, Altinors M, Karacan I. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath 2008;12:161-8.
- Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med 2012;185:715-22.
- Francescone RA, Scully S, Falbish M, Taylor SL, Oh D, Moral L, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem 2011;286:15332-43.
- Tang H, Fang Z, Sun Y, Li B, Shi Z, Chen J, et al. YKL-40 in asthmatic patients and its correlations with exacerbations, eosinophils and immunglobulin E. Eur Respir J 2010;35:757-60.
- R?ndjberg AK, Omerovic E, Vestergaard H. YKL-40 levels are independently associated with albuminuria in type 2 diabetes. Cardiovasc Diabetol 2011;10:54.
- Volck B, Johansen JS, Staltenberg M, Garbarsch C, Price PA, Ostergaard M, et al. Studies on ykl-40 in knee joints of patients with rheumatoid arthiritis and osteoarthiritis. Involvement of ykl-40 in the joint pathology. Osteoarthiritis Cartilage 2001;9:203-14.
- Th?m I, Andritzky B, Schuch G, Burkholder I, Edler L, Johansen JS, et al. Elevated pretreatment serum concentration of YKL-40-An independent prognostic biomarker for poor survival in patients with metastatic nonsmall cell lung carcinoma. Cancer 2010;116:4114-21.
- Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol 2000;32:911-20.
- Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, et al. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am. J Pathol 2008;173:130-43.
- Bhat KP, Pelloski CE, Zhang Y, Kim SH, deLaCruz C, Rehli M, et al. Selective repression of YKL-40 by NF-kappaB in glioma cell lines involves recruitment of histone deacetylase-1 and -2.? FEBS Lett 2008;582:3193-200.
- Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 2005;280:41213-21.
- Bonneh-Barkay D, Zagadailov P, Zou H, Niyonkuru C, Figley M, Starkey A, et al. YKL-40 expression in traumatic brain injury: an initial analysis. J Neurotrauma 2010;27:1215-23.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004;291:2013-6.
- Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003;107:1129-1134.
Yazışma Adresi (Address for Correspondence)
Dr. Serap DURU
Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi,
G?ğ?s Hastalıkları Kliniği, ANKARA - TURKEY
e-mail: akcalis@hotmail.com