RESEARCH ARTICLE
Doi: 10.5578/tt.9472
Tuberk Toraks 2015;63(3):147-157

Yoğun bakımda takip edilen sepsisli hastalarda ?oklu organ yetmezliği ve mortalite i?in risk fakt?rleri
Ece ?Z1, C?neyt SALT?RK2, Zuhal KARAKURT2, ?zlem YAZICIOĞLU MO?IN2, Nalan ADIG?ZEL2,
G?kay G?NG?R2, ?zkan DEVRAN3, Merih KALAMANOĞLU4, G?lbanu HORZUM2, Adnan YILMAZ2
1 Istanbul Medeniyet ?niversitesi Tıp Fak?ltesi, G?ğ?s Hastalıkları Anabilim Dalı, Istanbul, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Medeniyet University, Istanbul, Turkey
2 S?reyyapaşa G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi Eğitim ve Araştırma Hastanesi, Yoğun Bakım ?nitesi, Istanbul, T?rkiye
2 Medical Intensive Care Unit, Sureyyapasa Chest Diseases and Chest Surgery Training and Research Hospital,
Istanbul, Turkey
3 Yedikule G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği, Istanbul, T?rkiye
3 Clinic of Chest Diseases, Yedikule Chest Diseases and Chest Surgery Training and Research Hospital, Istanbul, Turkey
4 Kartal Koşuyolu Y?ksek Ihtisas Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği, Istanbul, T?rkiye
4 Clinic of Chest Diseases, Kartal Kosuyolu High Specialization Training and Research Hospital, Istanbul, Turkey
?ZET
Yoğun bakımda takip edilen sepsisli hastalarda ?oklu organ yetmezliği ve mortalite i?in risk fakt?rleri
Giriş: ?oklu organ yetmezliği (?OY) yoğun bakım ?nitelerinde ?nde gelen morbidite ve mortalite nedenlerinden biridir. Sepsis hastalarında ?OY gelişimine katkı sağlayan risk fakt?rlerinin tespit edilmesi ve ?nlenebilir problemlerin ??z?mlenmesi mortaliteyi azaltmada ?nemli bir adım olabilir. Bu ?alışmada yoğun bakımda yatan sepsis hastalarında ?OY ve mortalite ile bağlantılı risk fakt?rlerinin araştırılması ama?lanmıştır.
Hastalar ve Metod: Retrospektif veri toplamaya dayalı prognostik kohort ?alışması ger?ekleştirilmiştir. Ocak 2009-Mart 2010 tarihleri arasında, 22 yataklı solunumsal yoğun bakım ?nitesine sepsis tanısı ile yatırılan hastalar ?alışmaya dahil edilmiştir. Hastaların demografik verileri, Yoğun bakım ?nitesi (YB?) ciddiyet skorları, mekanik ventilasyon uygulaması, sepsise neden olan ajan, yoğun bakımda kalış s?releri ve mortalite varlığı kayıt edilmiş olup risk fakt?rleri i?in lojistik regresyon analizi uygulanmıştır.
Bulgular: ?alışmaya 347 sepsis hastası dahil edilmiştir. Kırk ?? hastada (%12.4) ?OY gelişmiş olup genel mortalite oranı %14.9'dur (n= 52). ?OY gelişimi i?in risk fakt?rleri diren?li patojen ve şok varlığı, total parenteral beslenme (TPB) ve y?ksek APACHE II skoru olarak bulunmuştur. (sırasıyla p= 0.015 Odds oranı (OR) 3.47 g?ven aralığı (CI): 1.27 - 9.47, p= 0.001, OR: 30.8 CI: 11.41 - 83 - 49, p= 0.028, OR: 3.08, CI: 1.13 - 8.39, p= 0.003, OR: 1.10, CI: 1.04 - 1.18). Genel mortalite risk fakt?rleri ise nozokomiyal infeksiyon varlığı, ???nc? g?n y?ksek SOFA skoru, şok varlığı, sedasyon ve TPB'dir. (sırasıyla p= 0.005, OR: 3.39, CI: 1.45 - 7.93; p = 0.001, OR: 1.51, CI: 1.27 - 1.81; p= 0.014, OR: 3.24, CI: 1.27 - 8.25; p= 0.003, OR: 3.64. CI: 1.54 - 8.58; p= 0.001, OR: 3.38, CI: 1.51 - 7.57).
Sonu?: Yoğun bakımda takibi gereken sepsis hastalarında diren?li patojen varlığı, şok, TPB uygulanması ve y?ksek APACHE II skoru ?OY gelişimi a?ısından risk fakt?rleridir. Bu nedenle akılcı antibiyotik kullanımı, TPB uygulamasının azaltılması, infeksiyon kontrol programlarının uygulanması ve şokun ?nlenmesi ?oklu organ yetmezliği ve mortaliteyi azaltacaktır.
Anahtar kelimeler: Yoğun bakım ?nitesi, ?oklu organ yetmezliği, sepsis
SUMMARY
Risk factors for multiorgan failure and mortality in severe sepsis patients who need intensive care unit follow-up
Introduction: Multiorgan failure (MOF) is a primary cause of morbidity and mortality in sepsis patients in intensive care units (ICU). Finding risk factors and solving preventable problems of MOF in patients who have sepsis can be a favourable step for decreasing mortality. We aimed to examine multiorgan failure and mortality related risk factors in intensive care unit patients who have sepsis.
Materials and Methods: A retrospective data collection and prognostic cohort study was performed. Between January 2009-March 2010, patients accepted to the 22-bed pulmonary intensive care unit with the diagnosis of sepsis were enrolled. Patients' demographic data, ICU severity scores, application of mechanical ventilation, causative agent of sepsis, number of ICU days and presence of mortality were recorded. Logistic regression analysis was carried out for risk factors.
Results: 347 patients with sepsis were involved in the study. 43 of the patients (12.4%) developed MOF and overall mortality rate was 14.9% (n= 52). Presence of resistant pathogen, presence of shock, application of TPN and high APACHE II score were found to be risk factors for MOF [p= 0.015 Odds ratio (OR) 3.47 confidence interval (CI): 1.27 - 9.47, p= 0.001, OR: 30.8 CI: 11.41 - 83-49, p= 0.028, OR: 3.08, CI: 1.13 - 8.39, p= 0.003, OR: 1.10, CI: 1.04-1.18, respectively]. Risk factors for overall mortality were presence of nosocomial infection, high 3rd day SOFA score, presence of shock, application of TPN and sedation (p= 0.005, OR: 3.39, CI: 1.45 - 7.93; p= 0.001, OR: 1.51, CI: 1.27 - 1.81; p= 0.014, OR: 3.24, CI: 1.27 - 8.25; p= 0.003, OR: 3.64. CI: 1.54 - 8.58; p= 0.001, OR: 3.38, CI: 1.51 - 7.57, respectively).
Conclusions: In sepsis patients who need ICU follow up, presence of resistant pathogen, presence of shock, application of TPN and high APACHE II scores are risk factors for developing MOF. Thus, rational use of antibiotics, reducing the use of TPN, application of infection control programmes and prevention of shock will further reduce multiorgan failure and mortality.
Key words: Intensive care unit, multiorgan failure, sepsis, mortality
INTRODUCTION
Sepsis is a major challenge in intensive care units (ICUs) as it was determined one of the leading causes of death (1). As early identification and treatment are essential, a "surviving sepsis campaign" has been implemented in the worldwide ICUs (2,3).
Multiorgan failure (MOF) is a major threat to the survival of patients with sepsis (4). It has been shown that patients with severe sepsis typically die due to MOF (5). In these patients, inappropriate activation of the immune system seems to contribute to organ dysfunction (6). Finding risk factors and overcoming preventable reasons of MOF in patients with sepsis may reduce mortality.
In this retrospective study, we primarily aimed to investigate MOF-related risk factors in patients with severe sepsis. Our secondary aim was to examine the factors affecting overall mortality in these patients.
Materials and Methods
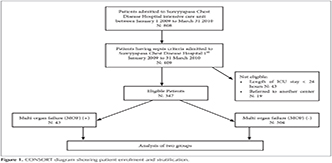
We designed a retrospective, observational, cohort study in a 20-bed, level III ICU of a tertiary teaching hospital for chest diseases between January 2009 and March 2010.? During the study period, a total of eight pulmonology specialists worked in the ICU, which they staffed 24 hours a day. This study was approved by the local ethics committee of Sureyyapasa Chest Diseases and Thoracic Surgery Teaching and Research Hospital, Istanbul, Turkey. Three hundred and forty seven patients admitted to the ICU with respiratory failure and sepsis were evaluated (Figure 1). All patients had acute respiratory failure and most of them had chronic obstructive pulmonary disease (COPD). All patients stayed in the ICU for more than 24 hour. In all patients, the etiology of sepsis was originated from a respiratory system disease. Patients were grouped according to having MOF or not and also to their survival status.
Demographic data, initial arterial blood gas (ABG) values, systemic inflammatory response syndrome (SIRS) criteria, acute physiological and chronic health evaluation (APACHE II) score, sequential organ failure assessment (SOFA) score, type of mechanical ventilation application, duration of invasive mechanical ventilation (IMV) or noninvasive mechanical ventilation (NIMV), length of ICU stay, the presence of septic shock,? need of insulin or sedative agent infusion, presence of a central venous catheter, need for total parenteral nutrition (TPN), and co-morbidities of the groups were recorded from patient files (1,7,8).
Definitions
Sepsis was defined as SIRS with a proven or suspected source of infection. SIRS was defined as presence of two or more of the SIRS criteria (1). Patients who had organ dysfunction and/or hypoperfusion abnormalities were defined as severe sepsis. Shock was defined as the need for vasoactive drugs ( > 5 μg/kg/min of dopamine or dobutamine or norepinephrine at any dose) for at least 1 hour (1). Septic shock was diagnosed when shock was associated with documented or assumed infection with no other identifiable cause (1). MOF was defined as the presence of altered organ function in an acutely ill patient such that homeostasis could not be maintained without intervention (9). Any organ function meeting the conditions below was considered to have dysfunction.
? Cardiovascular system: systolic blood pressure < 90 mmHg, mean arterial pressure MAP < 70 mmHg, signs of shock, ventricular tachycardia, ventricular fibrillation, or myocardial infarction.
? Respiratory system: hypoxia requiring respirator-assisted ventilation for at least 3-5 days or PaO2/FiO2 < 300 mmHg.
? Nervous system: indifference, restlessness, lethargy, light coma, or deep coma, Glasgow score ≤ 14 without sedation.
? Hematological system: prothrombin time (PT) and activated partial thromboplastin time (APTT) increase > 25% or platelets < 80.000 ? 109/L.
? Hepatic system: serum bilirubin levels ≥ 2-3 mg/dL, liver function tests ≥ twofold normal, international normalized ratio (INR) > 1.5.
? Renal system: creatinine levels > 1.4 mg/dL, urinary volume < 500 mL/24 h, or < 150 mL/8 h.
? Gastrointestinal system: intestinal ileus with intolerance to enteral feeding for > 5 days
Patients having more than one organ failure were considered to have MOF.
Modified Protocol for Surviving Sepsis
The "Early Goal-Directed Therapy" protocol was followed, which was based on fluid therapy to reach a MAP of 65 mmHg and appropriate antibiotic treatment (2,3).
Moderate tidal volume: providing a tidal volume not greater than 6 mL/kg per ideal body weight (10).
Moderate-dose steroids: stress-dose steroid therapy was applied in cases of septic shock after blood pressure was unresponsive to fluid and vasopressor therapy (basal cortisol or ACTH stimulation tests were not obtained as they were not available in our hospital) (11,12,13). Due to the absence of hydrocortisone in our country, methyl prednisolone was used at a dose of 20 mg tid for 7 days in patients without contraindications.
Glucose control protocol: when blood glucose level was > 150 mg/dL, continuous intravenous insulin infusion was applied to maintain blood glucose level between 110 and 140 mg/dL (< 150 mg/dL) (14,15).
Mechanical Ventilation
In our unit, noninvasive mechanical ventilation is the preferred method for ventilatory support of patients with acute respiratory failure if the patient has no contraindication (16). Invasive mechanical ventilation was applied with ICU ventilators (Prutan Bennett 760, Newport, Servo, Hamilton) if the patient had any of the intubation criteria, such as cardiac arrest, respiratory depression, loss of consciousness, hypercapnia unresponsive to NIMV, or shock. Assist control ventilation (A/C), with pressure or volume control, was preferred as the initial ventilation mode. In volume-control ventilation, inspiratory flow was set to provide an airway plateau pressure < 35 cmH2O and a tidal volume of 6-8 mL/kg ideal body weight was applied. In pressure-control ventilation, inspiratory pressure was set to apply a pressure of 30 35 cmH2O and titrated to reach a tidal volume of 6-8 mL/kg ideal bodyweight.
A weaning protocol was followed and patients meeting the weaning criteria were put on a t tube trial (17). Patients who were successful during 30-min t-tube trial were extubated.
Sedation
The Richmond agitation sedation (RAS) scale was used to determine the need for sedation. Continuous infusion of a sedative agent was avoided (18).
Laboratory Records
The complete blood count (CBC), serum biochemistry, and CRP levels of patients were recorded on their first day in the ICU. CBC and blood electrolytes were checked every day and CRP was checked daily and on the third day in the ICU (control). The SOFA score was calculated on the first and third days in the ICU and APACHE II was calculated on the initial and the discharge day. Initial arterial blood gases (ABGs) were recorded.
Microbiology
Bronchial secretions of the intubated patients were collected by deep tracheal aspiration into the tracheal aspirate tube. In non-intubated patients, sputum was collected into a sputum Petri dish. In cases of low or high fever (< 36?C or > 38?C), a blood sample was collected into aerobic culture medium. Bronchial lavage samples were taken from the patients who underwent bronchoscopy. Resistant pathogens were defined as microorganisms that were resistant to one or more therapeutic classes of antimicrobial agents (19).
Possible risk factors for MOF and mortality such as presence of shock, resistant pathogen, total parenteral nutrition, application of invasive mechanical ventilation, presence of nosocomial infection, development of second sepsis during ICU stay, presence of a central venous catheter, and body mass index were recorded. Demographic data,? departments where the patients were admitted, SIRS criteria, arterial blood gas values, number of days before ICU admission, first-day APACHE II, first- and third-day SOFA scores, sedative agents and insulin infusions, MV application, demographic data, and biochemical values of the both groups were compared.
Statistical Analysis
Continuous variables (age, biochemical values, body mass index, days on mechanical ventilation, APACHE II score, SOFA score, ICU days) with parametric and non-parametric values were analyzed by using Student's t-test and the Mann-Whitney U-test, respectively. Categorical variables (gender, co-morbidity, NIMV and IMV application) of both groups were analyzed by using the X2 test. A risk analysis for MOF was performed by using logistic regression analysis. All parameters found significant in univariate analysis and that may affect the development of multiorgan failure (age, gender, presence of shock, co-morbidities, total parenteral nutrition, days on IMV and presence of resistant pathogen) were added to the logistic regression analysis. Non-parametric values were expressed as medians and parametric values were expressed as means ? standard deviation. A p value < 0.05 was accepted as statistically significant.
Results
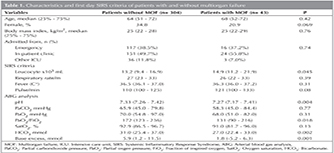
Three hundred and forty seven patients having sepsis criteria were included. Forty three patients (12.4%) had the multiorgan failure criteria. Of the 347 patients, 52 (14.9%) died in the ICU. Demographic data, initial arterial blood gas values, SIRS criteria, APACHE II score, SOFA score, type of MV application, MV days, number of days in ICU, presence of septic shock, insulin infusion, sedative agent infusion, presence of a central venous catheter, need for TPN, and co-morbidities of the groups were compared (Table 1). Leukocyte counts in patients with multiorgan failure were significantly higher than in patients without multiorgan failure (p< 0.045). pH (p< 0.004), PaO2/FiO2 (p< 0.018), HCO3 (p< 0.002), and base excess (p< 0.001) values in the first day arterial blood gas analysis of patients with multiorgan failure were significantly lower (Table 1).
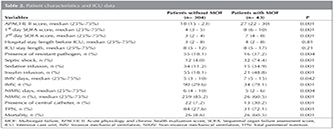
In Table 2, APACHE II scores, length of hospital stay before admission to ICU, length of ICU stay, presence of a nosocomial infection, presence of septic shock, TPN need, presence of a central catheter, sedation or insulin infusion, type of MV application, days on IMV and NIMV, and mortalities of patients with and without multiorgan failure are summarized. First day APACHE II first and third-day SOFA scores of patients with MOF were significantly higher than scores of patients without MOF (p= 0.001 for each; Table 2). Length of hospital stay before admission to ICU and length of ICU stay in both groups were similar (p> 0.05). Percentage of patients on IMV was higher for the group with MOF and IMV was applied for a longer time in this group when compared with the group without MOF (p= 0.001 and p= 0.042). The percentage of patients on NIMV was higher for the group without MOF and they were on NIMV for a longer time than the group with MOF (p= 0.001 and p= 0.004). Need for sedation (p= 0.001), insulin infusion (p= 0.001), presence of a central venous catheter (p= 0.001), need for total parenteral nutrition (p= 0.001), presence of shock (p= 0.001) and presence of nosocomial sepsis (p= 0.004) were significantly higher for patients with MOF versus patients without MOF (Table 2). The mortality rate was eightfold higher in the MOF group (Table 2).
For 174 (50.1%) patients, diagnostic procedures such as bronchial lavage, deep tracheal aspiration, blood and urine cultures were performed to identify the microorganism causing the sepsis. Resistant pathogen positivity was 55.8% (n= 24) for patients with MOF and 17.4% (n= 53) for patients without MOF (p= 0.001). The first three etiologic agents were Pseudomonas aeruginosa (n= 22, 28.5%), Acinetobacter baumannii (n= 20, 25.9%), and methicillin-resistant Staphylococcus aureus (MRSA; n= 10, 12.9%).
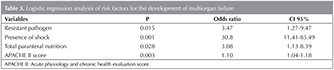
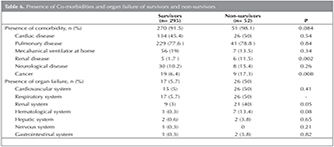
The incidences of renal disorders (p= 0.001) and malignancies (p= 0.035) were significantly higher in the MOF group. The presence of resistant pathogens, shock, sedation use, IMV application, insulin infusion, need for TPN, presence of nosocomial infection, PaO2/FiO2, APACHE II, and presence of renal disorders and malignancies were studied using a logistic regression model. The presence of resistant pathogens, shock, TPN, and high APACHE II scores were found as risk factors for the development of MOF. Although APACHE II score was statistically significant, (p = 0.003), its suggestibility was low due to the Odds ratio of 1.10 (Table 3).
Fifty two of the patients died and grouped as non-survivors. Demographic data and SIRS criteria were similar for survivors and non-survivors (Table 4). However, pH and PaO2/FiO2 values of non-survivors were significantly lower (p= 0.003 and p= 0.023, respectively).
APACHE II and SOFA scores, the presence of resistant pathogen, the application of mechanical ventilation, and the need for insulin, sedation and total parenteral nutrition for survivors and non-survivors are summarized in Table 5. APACHE II score, SOFA score and MV application were significantly higher in the non-survivor group (all p= 0.001).
Presence of co-morbidities and type of organ failure of survivors and non-survivors were shown in Table 6.
Nosocomial sepsis (p= 0.005), higher third-day SOFA score (p= 0.001), presence of shock (p= 0.014), sedation infusion (p= 0.003), and need for TPN (p= 0.003) were found to be related with mortality in logistic regression analysis (Table 7).
Discussion
In this study, we determined that the presence of a resistant pathogen, shock, the need for TPN and a high APACHE II score could be used to predict the development of MOF in ICU patients with respiratory failure along with severe sepsis. Besides this the presence of a nosocomial infection, shock, need for sedative agent infusion, TPN and high third-day SOFA score were also found to be predictors of overall mortality in the ICU.
A sepsis protocol that aims to provide immediate intervention to decrease mortality has been used widely. It has been reported that applying this protocol decreases mortality (20,21). However, some recent studies suggested conflicting results unlike the former ones as "Early Goal-Directed Therapy", was not found efficient in reducing the mortality of patients presenting with septic shock (22). In our study, we did not analyze the treatments which had been applied in other departments (emergency, ward and other ICU) before the patient was admitted to the ICU, although departments from which patients were admitted to the ICU were similar for both groups.
High blood glucose values and the need for insulin infusion indicated the severity of tissue injury which was caused by cytokines that play major role in the sepsis etiopathogenesis (6). However in a recent randomized controlled study there was no difference in the rate or severity of organ failure between the two different intensity of glycemic controls (23). Also in this study it was stated that cohort differences may play a role in assessing the impact of glycemia on organ failure. In our study the ratio of patients who needed insulin infusions were found significantly higher in the MOF group. Although we had standard target glucose level, there might be these kind of cohort differences in our study. Day in which insulin infusion started, frequency and amount of feeding, presence of insulin resistance, presence/dose of steroid therapy and comorbidities can affect the need for insulin infusion. It will be more accurate and externally valid to define effect of glycemic control on MOF in randomized clinical controlled studies.
IMV need is also already shown as a risk factor related with mortality. Significantly more patients were on IMV in the MOF group versus non MOF group. In contrast, the rate of NIMV application was significantly higher for patients without MOF. NIMV application is thought to decrease the need for IMV by protecting against the further problems that can be caused by IMV, so that it may prevent the development of MOF to some extent.
The first item of the sepsis protocol is "Early Goal-Directed Therapy", which must be applied in the first 4-6 h to prevent irreversible injury. In our study, first-day APACHE II and SOFA scores were markedly higher in MOF group patients versus than the non-MOF group (7,8). This may suggest that end organ damage had already begun and the opportunity to reverse the changes caused by sepsis had been missed, even the sepsis protocol was initiated without delay. Knox et al. stated that Glasgow Coma Scale scores dominated the association between admission SOFA score and 30-day mortality in a mixed ICU population (24). In our study, we did not analyze the contribution of every item of the SOFA score to mortality, but as our study included only patients treated in a respiratory ICU, the GCS part of the SOFA score may not be considered as a dominating item in SOFA scores of our patients.
Initiation of appropriate antibiotic treatment within 4-6 h after the sepsis process is considered essential (25,26,27). Tissue injury will be more severe in cases of resistant pathogens and inappropriate empirical treatment. A recent study showed that inappropriate antibiotic treatment increased the hospital mortality rate of patients with severe sepsis and septic shock (28). In our study microbiological examination was performed in half of our patients and more patients were infected significantly with a resistant pathogen in the MOF group, comparing with the group without MOF (72.7% and 37.6%, respectively). The low culture positivity was assumed to be due to ongoing antibiotic therapy at the time of specimen collection and the initiation of empirical therapy before collection of the culture specimen. Culture results revealed infection with multiple pathogens in most of the patients. P. aeruginosa, A. baumannii, and MRSA were the most commonly encountered pathogens for nosocomial sepsis.
There are studies that show suboptimal offer-demand nutrition in critically ill patients, which is characterized by deterioration of nutritional status, higher rates of multiple organ dysfunction, complications, cachexia, loss of muscle strength, prolonged length of stay, and mortality (29). Therefore, intensivists tend to feed the patient at any cost and they are prone to start TPN earlier than recommended. However it was clearly shown that TPN causes the translocation of bacteria into the gastrointestinal tract and increases the resistant pathogen population by decreasing obligate anaerobes, particularly in elderly patients with SIRS/sepsis (30,31). All other nutrition guidelines for critically ill patients including ESPEN, ASPEN, SCCM, and CCCPG agree with the benefits of early enteral feeding, and enteral feeding is superior to parenteral feeding. American Society for Parenteral and Enteral Nutrition (ASPEN) guideline recommends "If early enteral nutrition is not available in the first 7 days following admission to the ICU, no nutrition support therapy should be provided in the patient who was previously healthy prior to critical illness with no evidence of protein malnutrition; use of parenteral nutrition should be reserved and initiated only after the first 7 days of hospitalization (when enteral nutrition is not available) (32). In our center, we prefer not to use TPN unless essential. In our study population, all patients on TPN were suffering from gastrointestinal system bleeding. This likely explains its identification as a risk factor for MOF development.
As presence of shock plays an important role in the development of MOF, the sepsis treatment protocol targets immediate treatment of shock and thus preventing end organ damage. In this study, logistic regression analysis showed that the presence of shock increased the probability of MOF development by thirtyfold. Additionally, the MOF rate was increased threefold by total parenteral nutrition usage and having resistant pathogens. In the analysis of overall mortality risk factors, the presence of shock, sedative infusion, and nosocomial infection increased the mortality risk 3-3.5-fold and third-day SOFA score increased the risk 1.5-fold. A recent study stated that additional prognostic factors, such as age and co-morbidities, might complement the predictive performance of SOFA scores (33). In a study by Oltean et al. it was also reported that using a co-morbidity index might be beneficial in assessing the risk of death in septic patients (34). In our study, we analyzed the presence of co-morbidity as an independent risk factor for mortality. As most of our patients were elder and had COPD, almost all of them were accepted having co-morbidities. Renal disorders and malignancies were found significantly more frequent in the non-survivor group at comparison of comorbidities.
This study had several limitations. First, it was a retrospective study performed at a single center. However, the follow-up and treatment of patients like data collection were performed by the same physician group using the same optimized computer software; therefore significant valid data were gathered. Second, the study was carried out in a respiratory ICU and included only severe sepsis patients with a respiratory system origin. The results may differ somewhat if the population was different; however, it should also be noted that sepsis with a pulmonary origin constitutes half of all patients in general ICUs. Finally, we did not perform a microbiological examination in half of our patients. In our respiratory ICU, we routinely take endotracheal aspirate culture specimens from intubated patients. However, taking sputum examples is not always possible in patients who are not intubated, and contamination of these specimens by the oropharynx flora is also a big issue in these patients.
Conclusion
Risk of MOF development in sepsis patients can be reduced by taking some precautions. Early detection and appropriate treatment of resistant pathogens, accurate management of shock therapy, and reducing the use of TPN can lessen MOF development. Initial high APACHE II score should be an alerting parameter for MOF development.
CONFLICT of INTEREST
None declerad.
REFERENCES
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8.
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al; Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2000. Crit Care Med 2008;36:296-327.
- Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, Michalopoulos A, et al; SOAP Investigators.? Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care 2012;16:R222.
- Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med 2011;39:1050-5.
- Hotchkiss RS, Karl IE. The pathophysiology and management of sepsis. N Engl J Med 2003;348:138-50.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13;818-29.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendon?a A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8.
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 1999;27:723-32.
- Annane D, S?bille V, Charpentier C, Bollaert PE, Fran?ois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862-71.
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. CORTICUS Study Group. Hydrocortisone Therapy for Patients with Septic Shock. N Eng J Med 2008;358:111-24.
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al; NICE-SUGAR Study Investigators. Intensive versus conventional glucose control incritically ill patients. N Engl J Med 2009;360:1283-97.
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449-61.
- Majid A, Hill NS. Noninvasive ventilation for acute respiratory failure. Curr Opin Crit Care 2005;11:77-81.
- Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033-56.
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44.
- Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 1998;157:531-9.
- Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year Study. Intensive Care Med 2014;40:1623-33.
- van Zanten AR, Brinkman S, Arbous MS, Abu-Hanna A, Levy MM, de Keizer NF, et al; Netherlands Patient Safety Agency Sepsis Expert Group. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med 2014;42:1890-8.
- Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al; ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506.
- Penning S, Chase JG, Preiser JC, Pretty CG, Signal M, M?lot C, et al. Does the achievement of an intermediate glycemic target reduce organ failure and mortality? A post hoc analysis of the Glucontrol trial. J Crit Care 2014;29:374-9.
- Knox DB, Lanspa MJ, Pratt CM, Kuttler KG, Jones JP, Brown SM. Glasgow Coma Scale score dominates the association between admission Sequential Organ Failure Assessment score and 30-day mortality in a mixed intensive care unit population. J Crit Care 2014;29:780-5.
- Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med 2004;164:637-44.
- Metersky ML, Sweeney TA, Getzow MB, Siddiqui F, Nsa W, Bratzler DW. Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest 2006;130:16-21.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96.
- Vazquez-Guillamet C, Scolari M, Zilberberg MD, Shorr AF, Micek ST, Kollef M. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of outcome in severe sepsis and septic shock. Crit Care Med 2014;42:2342-9.
- Cahill NE, Dhaliwal R, Day AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: what is "best achievable" practice? An international multicenter observational study. Crit Care Med 2010;38:395-401.
- Steffen EK, Berg RD, Deitch EA. Comparison of translocationrates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis 1988;157:1032-8.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994;38:409-14.
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277-316.
- Lee KS, Sheen SS, Jung YJ, Park RW, Lee YJ, Chung WY, et al. Consideration of additional factors in Sequential Organ Failure Assessment score. J Crit Care 2014;29:185.e9-185.e12.
- Oltean S, Tatulescu D, Bondor C, Slavcovici A, Cismaru C, Lupşe M, et al. Charlson's weighted index of comorbidities is useful in assessing the risk of death in septic patients. J Crit Care 2012;27:370-5.
Yazışma Adresi (Address for Correspondence)
Dr. C?neyt SALT?RK
S?reyyapaşa G?ğ?s Hastalıkları ve G?ğ?s Cerrahisi
Eğitim ve Araştırma Hastanesi, G?ğ?s Hastalıkları Kliniği,
Yoğun Bakım ?nitesi, İSTANBUL-TURKEY
e-mail: csalturk@yahoo.com