ORIGINAL RESEARCH
Doi: 10.5578/tt.8538
Tuberk Toraks 2015;63(2):78-85

Uyku apne sendromu tan?s?nda g?nd?z polisomnografinin rol?
Fatih YAKAR1, Aysun YAKAR2, Murat SEZER1, Mustafa ERELEL3
1 Bezmi?lem Vak?f ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, ?stanbul, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Bezmi?lem Vakif University, Istanbul, Turkey
2 Adli T?p Kurumu, 2. ?htisas Kurulu, ?stanbul, T?rkiye
2 Forensic Science Institution, Ministry of Justice, Istanbul, Turkey
3 ?stanbul ?niversitesi ?stanbul T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, ?stanbul, T?rkiye
3 Department of Chest Diseases, Faculty of Istanbul Medicine, Istanbul University, Istanbul, Turkey
?ZET
Uyku apne sendromu tan?s?nda g?nd?z polisomnografinin rol?
Giri?: Uyku apne sendromu (UAS) tan?s? gece boyu s?ren polisomnografiyle (PSG) konmaktad?r, ancak test i?in bekleme s?resi olduk?a uzundur. G?nd?z a??r? uykululu?u bulunan hastalarda g?nd?z PSG yap?labilece?i ?nerilmi?tir ancak testin nas?l, ne kadar s?reyle yap?laca?? ve tan?daki rol? konusunda net bilgi yoktur. Bu ?al??man?n primer amac? g?nd?z yap?lacak PSG'nin uyku apne sendromu tan?s?n? koymadaki rol?n? saptamak, sekonder amac?ysa g?nd?z-gece PSG?leri aras?nda korelasyon olup olmad???n? belirlemektir.
Hastalar ve Metod: G?nd?z a??r? uykululuk hali olan ve uyku laboratuvar?na polismonografi yap?lmak ?zere g?nderilmi? 48 hasta cross-over ?al??maya al?nd?. Hastalar?n yar?s?na ?nce gece PSG daha sonra g?nd?z PSG yap?l?rken di?er gruba ?nce g?nd?z PSG daha sonra gece PSG yap?ld?. Yedi hasta PSG ?al??mas?na kat?lmad??? i?in ?al??madan ??kar?ld?. Polisomnografi kay?t ve analizleri AASM 2007 k?lavuzuna g?re ger?ekle?tirildi. Sonu?lar uyku etkinli?i, RDI, UAS a??rl???, oksijen desaturasyon indeksi (OD?), gece ve g?nd?z PSG aras?nda tan? korelasyonu a??s?ndan de?erlendirildi.
Bulgular: Toplamda 41 hasta analize al?nd?. T?m hastalara UAS tan?s? kondu. Gece PSG'de uyku etkinli?i daha y?ksek (%86) saptansa da g?nd?z PSG'de de yeterli etkinlik (%80) saptand?. Uykunun evreleri aras?nda gece PSG ve g?nd?z PSG aras?nda Evre 3 anlaml? fark yoktu (p> 0.05). Gece PSG'de daha fazla Evre 3 g?r?ld?. ?nce gece veya g?nd?z PSG yap?lmas?n?n uyku etkinli?i, RDI, OD? ve UAS?nin a??rl?yla ili?kisi olmad??? saptand?. V?cut kitle indeksi RDI, OD? ve UAS a??rl???yla ili?kili bulunmazken; boyun ?evresi yak?n ili?kili bulundu (p< 0.05).
Sonu?: G?nd?z PSG uygun ?ekilde yap?ld???nda, g?nd?z a??r? uykululu?u olan hastalarda UAS tan?s?n? koymada etkili bir y?ntemdir. G?nd?z PSG yap?larak hastalar?n test i?in bekleme s?releri azalabilir.
Anahtar kelimeler: Uyku apne sendromu, tan?, g?nd?z, polisomnografi
SUMMARY
Role of daytime polysomnography in the diagnosis of sleep apnea syndrome
Introduction: Diagnosis of sleep apnea syndrome (SAS) depends on nocturnal polysomnography (nPSG), but waiting time for the test is long. Although, performing PSG in patients with excessive daytime sleepiness (EDS) at daytime (dPSG) was questioned, role and methods are not clear. The aim of the study was to assess the role of dPSG in the diagnosis of SAS, and the correlation between nPSG and dPSG.
Patients and Methods: Forty eight subjects, who were referred to our sleep laboratory with EDS were included to a cross-over study. Half of the patients underwent nPSG after dPSG, vice versa. Seven subjects excluded due to lack of participation. The rest (n= 41) had nPSG and dPSG. PSG recordings and analysis were performed according to AASM 2007 guideline. Results were analyzed for sleep efficiency, respiratory disturbance index (RDI), oxygen desaturation index (ODI), SAS severity and correlation between dPSG and nPSG.
Results: Total 41 subjects were analyzed. All patients had diagnosis of SAS. Sleep efficiency was higher at nPSG (86%), however also enough at dPSG (80%). Sleep stages of dPSG and nPSG were similar except stage 3 sleep, which was longer at nPSG. Undergoing dPSG or nPSG first did not correlate with sleep efficiency, respiratory disturbance (RDI) index, oxygen desaturation index (ODI) or severity of SAS. Despite BMI, neck circumference was closely related with RDI, ODI and severity.
Conclusion: Daytime PSG, when performed appropriately, is an effective tool for diagnosing sleep disorders in patients with EDS. dPSG may decrease the amount of times that patients must wait to undergo PSG.
Key words: Sleep apnea syndrome, polysomnography, daytime diagnosis
INTRODUCTION
Sleep apnea syndrome (SAS) is a disorder with an increasing prevalence and well-established neurocognitive dysfunction and cardiovascular sequelae (1,2). Although debate is ongoing, Young et al. reported that the prevalence of SAS was 3-28% (3,4). Older patients and African-Americans are more prone to the disorder (5).
Several tests and questionnaires can be used for the diagnosis of the SAS, but the gold standard remains nocturnal polysomnography (nPSG). The main problem with PSG is accessibility, because PSG is not available at every medical center and requires experienced technicians and physicians. Flemons et al. reported that non-emergency patients may have to wait 14 (7-60) months for PSG in England (6). Similarly, the waiting time for the test at our center is 14 months, which delays diagnosis and treatment.
Excessive daytime sleepiness (EDS) is a frequent symptom of SAS patients. It may affect jobs requiring attention and may cause reduced productivity, motor vehicle accidents, industrial accidents, or work-related injuries generally (7). Previous studies have shown that patients with EDS may sleep during the daytime and are suitable for short periods of PSG evaluation (8,9). However, the validity, role, and appropriate techniques fordaytime PSG (dPSG) in the diagnosis and management of SAS are unclear.
The aim of the study was to assess the role of daytime PSG in the diagnosis and evaluation of SAS, and the correlation between nPSG and dPSG.
PATIENTS and METHOD
Patients
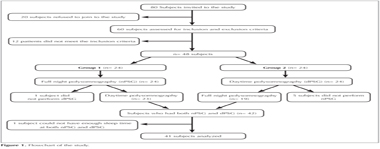
We recruited consecutive patients referred to our laboratory with complaints of snoring and EDS over a 12-month period. Eighty subjects were invited to participate in the study; 20 refused to participate. Sixty subjects who were willing to participate were invited to the sleep laboratory and evaluated for exclusion criteria by a sleep physician. Twelve subjects were excluded, leaving 48 who participated in the study. The inclusion and exclusion criteria are presented in Table 1.
Local Ethics Committee approval was obtained.Written informed consent was provided by all subjects.
Study Design
We conducted a prospective, cross-over study at the sleep laboratory of a university hospital over a 12-month period. Demographic characteristics, body mass indices (BMI), neck circumference, Epworth sleepiness scale (ESS), co-morbidities, and alcohol, drug, and smoking histories were recorded before the study. All subjects planned to undergo both 7-hours nPSG and 7-hours dPSG and were randomized into two groups: the first had nPSG before dPSG, while the other group had dPSG before nPSG (Figure 1). Patients were warned to not be sleep-deprived before the PSG sessions. The time interval between the two PSGs was 48-72 h. nPSG was performed from 00:00-07:00 hours and dPSG from 10:00-17:00 hours.
Polysomnography
A Compumedics E-series with Profusion software was the standard PSG machine. All measurements and assessments were performed according to the AASM 2007 guidelines (10). Pulse oximetry, C3-A2, C4-A1, O1-A2, and O2-A1 electroencephalograms, electrooculograms, electromyograms (mentalis and legs), and electrocardiograms were recorded, and respiration was monitored using a nasal cannula, oronasal thermistor, and thoracoabdominal piezo sensors in dPSG and nPSG. Polysomnography was performed with strict control of the environment. Only the technician was present in the laboratory during PSG. Cleaning of the laboratory was performed outside of study hours. All cell phones and other electronic devices were switched off. The temperature was fixed at 22-24?C by means of air conditioning. Daylight was eliminated using thick, dark curtains.
Scoring
Polysomnography data were scored and analyzed by two experienced and blinded sleep physicians. Sleep stages and events were scored according to the AASM 2007 guidelines (10). Apnea was defined as cessation of airflow for ≥ 10 s, and hypopnea was defined as a > 50% reduction in airflow accompanied by oxygen desaturation of ≥ 3% for ≥ 10 s. The respiratory disturbance index (RDI) was defined as the average number of episodes of apnea, hypopnea, and respiratory event-related arousal per hour of sleep. Sleep stages, sleep efficiency, total sleep time (TST), RDI, oxygen desaturation index (ODI), lowest oxygen saturation (SpO2), and time period of oxygen saturation < 90% (SpO2 < 90%) were analyzed. Individuals with an RDI ≥ 5 were considered to have SAS, which was classified as mild (RDI 5-15), moderate (RDI 16-30), or severe (RDI ≥ 30). Obesity hypoventilation syndrome (OHS) was defined as BMI ≥ 30 kg/m2, chronic daytime hypercapnia (PaCO2 < 45 mmHg), and RDI ≥ 5/h in the absence of other known causes of hypercapnia (11).
Statistical Analysis
Demographic characteristics, BMI, neck circumferences, PSG parameters and results, ESS scores, and co-morbidities were analyzed using Wilcoxon signed-rank tests, paired t-tests, Spearman's rho and the Mann-Whitney U-test with the IBM SPSS software (ver. 20).
Results
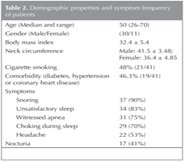
In total, 48 subjects were included in the study. Six subjects did not participate in nPSG or dPSG PSG and one subject did not have sufficient sleep time at the PSG sessions. These seven subjects were excluded; therefore, data for 41 subjects (30 males, 11 females) were analyzed (Figure 1). Their median age was 50 (26-70) years and their mean BMI was 32.4 ? 5.4. Nineteen patients (46.3%) had one or more comorbidities (hypertension, diabetes, or coronary artery disease), but there was no correlation between comorbidities and SAS severity (p = 0.46). Demographic characteristics and symptoms are provided in Table 2. The mean ESS score was 11.3 ? 5.94. There was no correlation between ESS score and symptoms (p< 0.05) but not with comorbidities (p> 0.05). Twenty one patients were current or former smokers, but there were no correlations between smoking and sleep parameters, BMI, or neck circumference (p> 0.05).
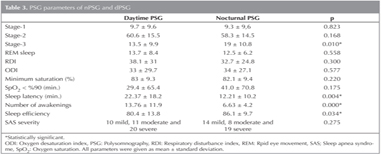
Sleep stages in nPSG and dPSG were similar, with the exception of stage 3. The stage 3 sleep ratio was higher in nPSG than dPSG (dPSG: 13.5 ? 9.9% vs. nPSG: 19 ? 10.8%; this difference was statistically significant, p= 0.01). RDI, ODI, minimum saturation, TST, and the time period of SpO2 < 90%, did not differ significantly between dPSG and nPSG. Although the duration of SpO2 < 90% was longer in nPSG than dPSG (41.0 ? 70.8 vs. 29.4 ? 65.4), the difference was not statistically significant (p= 0.17). Sleep efficiencies in nPSG and dPSG were 86% ? 9.7 and 80% ? 13.8, respectively (mean ? SD).This difference was statistically significant (p= 0.034), but there was no correlation between dPSG and nPSG efficiencies (p> 0.05) (Table 3). Undergoing dPSG or nPSG first did not correlate with sleep efficiency, RDI, ODI, or severity of SAS (p> 0.05).
Sleep latencies of the patients in nPSG and dPSG were 12.2 ? 10.2 and 22.3 ? 18.2, respectively (p< 0.005). Awake periods were less common in nPSG than dPSG (6.6 ? 4.2 vs. 13.7 ? 11.9, p< 0.001). Both differences were statistically significant, favoring nPSG.
Treatment plans for the patients were developed according to the nPSG results. Using nPSG, we diagnosed 14 mild, 8 moderate, and 19 severe SAS patients. dPSG revealed 10 mild, 11 moderate, and 20 severe SAS cases; the difference was not statistically significant (p= 0.275). If the patients were categorized by positive airway pressure (PAP) requirement (mild vs. moderate and severe), dPSG showed a higher PAP requirement than nPSG, but the difference was not statistically significant (p> 0.05).
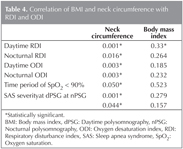
Mean neck circumference was 40.29 ? 4.1 cm (males: 41.5 ? 3.48 cm, females: 36.4 ? 4.85 cm). There were positive correlations between neck circumference and nocturnal RDI and daytime RDI (p< 0.001), ODI (p< 0.05), severity of SAS (p< 0.001), and duration of SpO2 < 90% (p< 0.05) (Table 4). However, there was no correlation between ESS and neck circumference (p= 0.69).
Mean BMI was 32.4 ? 5.4 km/m2. There were no correlations between BMI and daytime RDI and ODI, nocturnal RDI and RDI, and time period of SpO2 < 90% (p> 0.05) (Table 4).
Discussion
In this study, we showed that dPSG is an effective method for diagnosing SAS and that the difference between dPSG and nPSG parameters was not statistically significant. RDI, ODI, minimum saturation, and duration of saturation < 90% did not differ between nPSG and dPSG (p> 0.05).
Polysomnography during the daytime was used previously in various ways. Goode and Slyter examined 310 patients, diagnosed 102 cases of obstructive sleep apnea syndrome (OSAS) using dPSG, and reported dPSG to be a useful test (12). Mizuma et al. showed a positive correlation between dPSG and nPSG; however, they performed sleep deprivation before dPSG, which may have increased the number of apnea and hypopnea events (9). Persson et al. and Desai et al. showed that sleep deprivation increased the RDI, total sleep time, sleep efficiency, and REM and stage 3 sleep (13,14). In this study, we did not perform sleep deprivation before either PSG session; however, we cannot eliminate the possibility of intentional sleep deprivation by patients to achieve better test results.
Some studies of the value of daytime PSG assessed less than 2h of sleep and reported that dPSG was not useful without nPSG (15,16,17). However, these studies did not include sufficient sleep time to evaluate dPSG. Fourre et al. reported that REM sleep did not occur during dPSG, but we found no difference in REM sleep ratios between dPSG and nPSG (16).
Yoshiko and Okada reported dPSG to be a useful screening test with significant false negativity (18). Van Keimpema reported 88% specificity and 66% sensitivity (17). However, Miyata et al. reported greater specificity (100%) and sensitivity (81%), likely due to increased TST (19). In our study, we performed 7-h dPSG and nPSG. All subjects were diagnosed with SAS by both dPSG and nPSG, probably because only patients with EDS were included in the study. However, excluding patients due to lack of participation or inadequate sleep time may have affected the results.
Sleep efficiency depends primarily on the patient and environment. Le Bon et al. reported a first-night effect of 15-25% in sleep studies, which could lead to misdiagnoses (20). This first-night effect may be related to both patients' habits (bed, pillow, room change) and discomfort due to the PSG equipment. However, sleep efficiency in our study was high (nPSG: 86 ? 9.7% and dPSG: 80 ? 13.8%), likely due to our strict environmental controlsfor provision of sleep hygiene. Similar results were reported by Miyata et al. (nPSG 73.3% and dPSG 68.1%). According to the correlation analysis, performing nPSG or dPSG first did not affect the PSG parameters or the results (p>0.05).
Sleep stages (except stage 3) and total sleep time were similar in dPSG and nPSG; however, stage 3 sleep (p< 0.05), sleep latency (p< 0.05), and awakenings (p< 0.001) differed significantly. The decreased ratio of stage 3 sleep, requirement for a longer time to fall a sleep, and more frequent awakenings during dPSG may indicate shallower sleep. Although there was a statistically significant difference, it did not affect the diagnosis, the severity of the sleep problems, or cause any clinical difference.
Most patients admitted to sleep laboratories are overweight or obese. Al-Jawder et al. reported a 33.1% OHS frequency in a daytime PSG study (21). However, only four patients had OHS in our study, which might have been due to our relatively less obese population. It is known that as BMI increases, RDI increases; however, neck circumference is a better predictor and correlatesbetter with the severity of sleep apnea than BMI (22,23,24). Although our subjects had neck circumferences lower than the upper limits, neck circumference was correlated with RDI and ODI in nPSG and dPSG (p< 0.05), but BMI was not (p> 0.05) (Table 2). This finding is consistent with previous reports.
The Epworth sleepiness scale assesses the effects of sleep problems on daily life. Patients were categorized as sleepy or non-sleepy according to their ESS scores (25,26). In our study, ESS did correlate with symptoms (p< 0.05) but did not correlate with comorbidities, SAS severity, lowest saturation, duration of SpO2 < 90%, sleep stage, or PSG efficiency (p> 0.05). This was likely due to the subjective nature of the test, because the education level of the subjects and characteristics of the population may affect the overall scores.
The average waiting time for PSG in Turkey is nearly 1 year (7-12 months). This can lead to patients contacting multiple sleep laboratories in an attempt to undergo PSG at an earlier date. However, multiple admissions may cause unreal laboratory load. Unfortunately, the current social security system in Turkey does not prevent multiple admissions. However, performing dPSG may increase the laboratory capacity, efficiency, and shorten waiting lists.
Although dPSG is simple to perform, the sleep technicians must be careful to control the environment. Another problem with the dPSG is the possibility of affecting the life style of the subjects due to having many hours at the laboratory during daytime (i.e having lunch).
There were several limitations to this study. First, although we recruited referred patients consecutively and did not perform any gender selection, a greater number of male subjects were included. This was probably due to the male preponderance of SAS (3). Second, our study population comprised relatively young subjects, which might explain the scarcity of comorbidities as these increase with advancing age. Third, information regarding the sleep habits and occupations of the subjects was lacking. Occupation can affect sleep habits. People who work night shifts (night watchman, taxi drivers) may normally sleep during the day. These sleep habits may decrease the efficiency of nPSG and exaggerate the efficiency of dPSG. Fourth, a small number of subjects were included, which this was due to the strict exclusion criteria. Although we have started with 80 participants, 39 were eliminated based on the exclusion criteria, insufficient sleep time, or unwillingness to participate. Also, the study concerned only patients with EDS; therefore, the results cannot be generalized to all sleep apnea patients.
Conclusion
Daytime PSG, when performedappropriately (patient selection and environment control), is an effective tool for diagnosing sleep disorders in patients with EDS. dPSG may increase the capacity of sleep laboratories and decrease the amount of times that patientsmust waitto undergo PSG.
Acknowledgement
None of the authors declares any conflict of interest.
CONFLICT of INTEREST
None declared.
REFERENCES
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet 2002;360:237-45.
- Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis 2009;51:313-23. doi:10.1016/j.pcad.2008.02.003.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J of Med 1993;328:1230-5. doi:10.1056/nejm199304293281704.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-39.
- Silber MH, Krahn LE, Morgenthaler TI. The parasomnias. In: Silber MH, Krahn LE, Morgenthaler TI (eds). Sleep Medicine in Clinical Practice. London:Taylor and Francis, 2004:321-345.
- Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 2004;169:668-72. doi:10.1164/rccm.200308-1124PP
- Colten HR, Altevogt BM. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC):National Academies Press, 2006.
- Mahakit P. A comparative study of two-hour daytime and overnight polysomnography in high risk snorers. J Med Assoc Thai 2002;95(Suppl 5):S17-S22.
- Mizuma H, Sonnenschein W, Meier-Ewert K. Diagnostic use of daytime polysomnography versus nocturnal polysomnography in sleep apnea syndrome. Psychiatry Clin Neurosci 1996;50:211-6.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine, 2007.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
- Goode GB, Slyter HM. Daytime polysomnogram diagnosis of sleep disorders. J Neurol Neurosurg Psychiatry 1983;46:159-161. doi:10.1136/jnnp.46.2.159
- Persson HE, Svanborg E. Sleep deprivation worsens obstructive sleep apnea. Comparison between diurnal and nocturnal polysomnography. Chest 1996;109:645-50
- Desai AV, Marks G, Grunstein R. Does sleep deprivation worsen mild obstructive sleep apnea? Sleep 2003;26:1038-41.
- Scharf SM, Garshick E, Brown R, Tishler PV, Tosteson T, McCarley R. Screening for subclinical sleep-disordered breathing. Sleep 1990;13:344-53.
- Fourre J, Scoles V, Nahmias J, Karetzky MS. The use of nap studies in diagnosing obstructive sleep apnea. Sleep Res 1986;15:122.
- van Keimpema AR, Rutgers SR, Strijers RL. The value of one hour daytime sleep recording in the diagnosis of the sleep apnoea syndrome. J Sleep Res 1993;2:257-9.
- Yoshiko K, Okada T. [Screening of sleep apnea syndrome using daytime polysomnography--its usefulness and the characteristics of daytime sleep in the patients with sleep apnea syndrome]. Rinsho Byori 1993;41:279-84.
- Miyata S, Noda A, Nakata S, Yagi H, Yanagi E, Honda K, et al. Daytime polysomnography for early diagnosis and treatment of patients with suspected sleep-disordered breathing. Sleep Breath 2007;11:109-15. doi:10.1007/s11325-006-0091-9.
- Le Bon O, Hoffmann G, Tecco J, Staner L, Noseda A, Pelc I, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest 2000;118:353-9.
- Al-Jawder S, Bahammam A. Utility of daytime polysomnography for in-patients with suspected sleep-disordered breathing. Neurol Neurochir Pol 2009;43:140-7.
- Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Sleep Heart Health Study. "Methods for obtaining and analyzing unattended polysomnography data for a multicenter study". Sleep Heart Health Research Group. Sleep 1998;21:759-67.
- Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J 1992;5:377-81.
- Santaolalla Montoya F, Iriondo Bedialauneta JR, Aguirre Larracoechea U, Martinez Ibarguen A, Sanchez Del Rey A, Sanchez Fernandez JM. The predictive value of clinical and epidemiological parameters in the identification of patients with obstructive sleep apnoea (OSA): a clinical prediction algorithm in the evaluation of OSA. Eur Arch Otorhinolaryngol 2007;264:637-43.
- Smith SS, Oei TP, Douglas JA, Brown I, Jorgensen G, Andrews J. Confirmatory factor analysis of the Epworth Sleepiness Scale (ESS) in patients with obstructive sleep apnoea. Sleep Med 2008;9:739-44. doi:10.1016/j.sleep.2007.08.004.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5.
Yaz??ma Adresi (Address for Correspondence)
Dr. Fatih Yakar
Bezmi?lem Vak?f ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
?STANBUL - TURKEY
e-mail: fyakar@yahoo.com