RESEARCH ARTICLE
Doi: 10.5578/tt.3138
Tuberk Toraks 2014;62(4):279-285

Akci?er kanseri hastalar?n?n preoperatif de?erlendirmesinde? vibration response imaging cihaz?n?n yeri
Nurdan ??M?EK VESKE1, Sinem Nedime S?K?C?1, G?l?ah G?NL?O?LU1, Ekrem Cengiz SEYHAN1, Levent DALAR2,
Sedat ALTIN2
1 ?stanbul Yedikule G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi, G???s Hastal?klar? Klini?i,
?stanbul, T?rkiye
1 Clinic of Chest Diseases, Istanbul Yedikule Chest Diseases and Chest Surgery Training and Research Hospital,
Istanbul, Turkey
2 ?stanbul Yedikule G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi, Solunum Yo?un Bak?m ?nitesi,
?stanbul, T?rkiye
2 Respiratory Intensive Care Unit, Istanbul Yedikule Chest Diseases and Chest Surgery Training and Research Hospital,
Istanbul, Turkey
?ZET
Akci?er kanseri hastalar?n?n preoperatif de?erlendirmesinde? vibration response imaging cihaz?n?n yeri
Giri?: ?al??mam?zda, cerrahi rezeksiyon planlanan k???k h?cre d??? akci?er kanserli hastalar?n preoperatif de?erlendirilmesinde ikinci basamak testi olarak vibration response imaging (VRI) kullan?m?n?n ve predicted postoperatif zorlu ekspiratuar vol?m 1. sn (PPO FEV1) tahminindeki yerini ara?t?rmay? ama?lad?k.
Materyal ve Metod: Operasyon planlanan ve ?al??may? tamamlayan toplam 31 hastaya postoperatif akci?er fonksiyonlar?n? tahmin etmek amac?yla solunum fonksiyon testleri (SFT), VRIxp ve kantitatif akci?er perf?zyon sintigrafisi yap?ld?. Postoperatif 4-6. haftalarda tekrar SFT ve VRI yap?larak postoperatif FEV1 ve % FEV1 de?erlerini cerrahi ?ncesi VRI ve perf?zyon sintigrafisiyle hesaplanan ppo-FEV1 ile kar??la?t?r?ld?. Postoperatif takibi olan 31 hastan?n verilerinin istatistiksel analizi iki b?l?mde yap?ld?. ?lk b?l?mde operasyon ?ncesi iki tahmin etme metodu (perf?zyona dayanan ppo ile VRI ile hesaplanan ppo), ikinci b?l?mde ise tahmini verilerle operasyon sonras? elde edilen ger?ek de?erler (postoperatif spirometrik de?erler standart kabul edilerek) kar??la?t?r?ld?.
Bulgular: VRI ile hesaplanan ppo de?eriyle perf?zyonla hesaplanan ppo aras?nda uyum oran? %52 idi. Bu oran d???k olarak saptansa da, tahmini ppo VRI ve ppo perf?zyon de?erleri i?in kestrim de?eri s?ras?yla %84 ve %47'dir. Bu da g?stermektedir ki ?al??mam?za al?nan hastalarda 1. ay postoperatif de?eri tahmin etmede, ppo VRI, kantitatif perf?zyon sintigrafisinden daha anlaml? bulunmu?tur.
Sonu?: VRIxp, postoperatif FEV1 tahmininde y?ksek do?ruluk oran?na sahiptir. Yatak ba?? uygulama kolayl??? olmas?, radyasyonsuz ve noninvaziv olu?u nedeniyle de preoperatif de?erlendirmede kantitatif perf?zyon sintigrafisine alternatif gibi g?r?lmektedir.
Anahtar kelimeler: Akci?er kanseri, preoperatif de?erlendirme, vibration response imaging, kantitatif perf?zyon sintigrafisi
SUMMARY
Place of vibration response imaging in preoperative lung cancer patients
Introduction: In this study, we aimed to investigate the role of the vibration response imaging (VRI) as the second-line test in preoperative evaluation of the patients with non-small cell lung cancer (NSCLC) and in prediction of the predicted postoperative forced expiratory volume in one second (ppo FEV1).
Materials and Methods: A total of 31 patients scheduled for surgery underwent pulmonary function tests (PFTs), VRIxp and quantitative lung perfusion scintigraphy (LPS) in order to predict postoperative lung functions. PFTs and VRI were repeated between 4th and 6th postoperative weeks and FEV1 and FEV1% values were compared with preoperative VRI and ppo-FEV1 calculated with perfusion scintigraphy. Statistical analysis of 31 patients under postoperative follow-up was performed in two parts. In the first part, two preoperative prediction methods (ppo based on perfusion and ppo calculated with VRI), and in the second part, estimated values and postoperative actual values (considering postoperative spirometric values as standard) were compared.
Results: An agreement rate of 52% was found between the ppo values calculated with VRI and with perfusion. This rate was low, although respective predictive values for ppo VRI and ppo perfusion were 84% and 47%, suggesting that ppo VRI was more significant than LPS for prediction of the 1st month postoperative value in the patients included in this study.
Conclusions: VRIxp has a high-accuracy rate in prediction postoperative FEV1. It is seemed as an alternative to quantitative perfusion scintigraphy for preoperative evaluation, because it can be easily applied at the bedside as a radiation free and non-invasive method.
Key words: Lung cancer, preoperative evaluation, quantitative lung perfusion scintigraphy, vibration response imaging
INTRODUCTION
Lung complications developed in the postoperative period are an important cause of morbidity and mortality in the patients undergoing resection due to lung cancer. Therefore, lung functions remaining after the surgery are calculated in these patients to predict the risk for respiratory failure. Thus, extent of the resection which might be tolerated by the patient can be decided. However, none of the tests used for preoperative evaluation enables a definite contraindication decision for the surgery. These tests only provide us to make a prediction about the risks for postoperative mortality and complications.
Algorithm protocols of pulmonary functions must be settled, and new imaging methods must be developed in the patients with pulmonary functions at the borderline. Recently developed Vibration Response Imaging xp (VRIxp) test produces dynamic and functional lung images by recording the vibration created by the airflow moving in to the lungs. VRIxp was designed to determine the status of lung ventilation and to provide a better understanding of lung functions in relation with the respiratory cycle (1,2).
In this study, we aimed to investigate the role of vibration response imaging device in patients with lung cancer and to compare this test with quantitative perfusion scintigraphy among the second-line tests.
MATERIALS and METHODS
Consecutive patients (with baseline FEV1% below 40) diagnosed with non-small cell lung cancer, scheduled for lobectomy or pneumonectomy following metastasis screening (thorax CT, FDG-PET/CT, brain MR) and with the requirement of quantitative perfusion scintigraphy as the second-line test were included in the study between October 2008 and April 2009. Total 46 patients were enrolled in the study. Of these patients, 4 were excluded due to positive N2 lymph node detection in mediastinoscopy and 9 patients due to refusing to be operated. PFTs, VRI and perfusion scintigraphy were performed in all the patients. One of 33 operated patients was lost due to massive hemoptysis at 20th postoperative day, and one patient was taken to the intensive care unit at the 3rd postoperative hour because of ventricular fibrillation and became exitus at the 29th day of follow-up in the ICU. Thus, 31 of 46 patients undergone PFTs, VRI and quantitative perfusion scintigraphy at the beginning completed the study and constituted the study group.
Inclusion criteria of the study were to be in between 18-85 years of age with a body mass index > 19, being able to make regular inspirium and expirium during the use of VRI device, not having hirsutism and chest wall or spinal deformity, absence of potentially contaminating skin lesions in the back, not having cardiac pacemaker or implantable defibrillator (1), absence of active pulmonary infections, decompensated cardiac and chronic airway diseases and being able to clean the secretions. The study was approved by the Hospital Ethics Committee. The patients were informed about the study and consent forms were received from all the participants.
The patients were questioned for demographic features, known diseases, medications used, smoking and previous operations. Body mass index was defined as the body mass divided by the square of height in meters (kg /m?).
Pulmonary function testing (PFT) was performed in the pulmonary function laboratory of our hospital according to ATS/ERS criteria by a specially trained technician using a spirolab III spirometer device (3). Postoperative measurements were carried out by the same team. Macro aggregated albumin (MAA) radionuclide perfusion study was preoperatively performed in all the patients using a multidetector system. All the patients were taken to VRI study on the same week of this process. Postoperative measurements (pulmonary function tests and VRI) were carried out between the 4th and 6th weeks after the surgery.
Device Properties
VRIxp device (Deep BreezeTM, Or-Akiva, Israel) is a non-invasive device-based computer system which provides regional quantitative data and dynamic lung images obtained by recording the vibrations created during the respiratory circle (1,2). This device includes monitor, keyboard, main memory and 6 or 7 rows of 2 sensors and recorder at the right and left sides (Figure 1).
Patient Preperation
Metal accessories on the patient were removed before the process. Back of the patient and surface of the sensors were wiped with ethanol. The patients were provided to sit in a proper position (with their back side free, tilted slightly forward, arms in a comfortable position between the legs, facing the device) The patients were trained about respiratory circle practice (to breathe deeply but not forced from the mouth, without moving their body and shoulders, only having their chest to rise and fall). The patients were provided to make few practices of the respiratory circle before the recording.
Application of the Test
The tests were applied in a silent setting in order to prevent the sensors to detect any noise might interfere from the outer environment. The sensors were used as to the height of the patients (7-sensors for > 165 cm; 6-sensors for < 165 cm). The sensors were inserted in an equal distance on both sides of the vertebra with 3-6 cm intervals and the upper end to be located at 1.5 cm above the scapula (Figure 1). The patients were provided to breathe with the same amplitude and frequency for 12 seconds during the recordings. Once the three VRIxp recordings for a patient were uploaded to the Q-Plan software, the physician then selected the maximum number of good-quality breathing frames to be added to the average quantitative lung data (QLD) calculations for each recording. Average QLD and standard deviation (SD) were then calculated by the software to produce the final average QLD value. The SD was used to ensure the reliability of the data; a value of the SD below 5.4 was chosen as the acceptable limit. Preoperative lung function data were entered into the software, through the user interface, and the intended surgical site was selected on the Q-Plan lung resection diagram. The predictions were calculated as published equations (4-6).
Statistical Analysis
Statistical analyses of 31 patients with postoperative follow-up were performed in two parts. In the first part, two preoperative prediction methods (ppo based on perfusion and ppo calculated with VRI), and in the second part, estimated values and postoperative actual values (considering postoperative spirometric values as standard) were compared. Pearson's correlation was used to evaluate the correlation between ppo values from VRI and perfusion screening in the patients with pneumonectomy; Wilcoxon signed test to determine the difference between the matched data and Bland- Altman's method was used to evaluate the agreement between the postoperative actual values of VRI and perfusion scintigraphy.
RESULTS
Parameters of the pulmonary function test were analyzed in 31 patients with postoperative VRI and PFTs follow-up (mean age 58.35 ? 10.7 years; range 34-77; 28 males). The tumors were found to be localized in left hilar (4), right hilar (3), left upper lobe (5), right lower lobe (4), left lower lobe (4) and right upper lobe (11). Adenocarcinoma (6), squamous cell (7), carcinoid (1), large cell (1) and non-small cell carcinomas (16) were diagnosed as the histological types.
Twenty patients undergone lobectomy and 11 pneumonectomy; ppo FEV1% calculated with VRIxp and ppo FEV1% calculated with quantitative perfusion scintigraphy were found as %57 ? 21 and 51% ? 18; respectively. Average delta between the two methods was %5.8 ? %10.6. Pearson's correlation was calculated as 0.8697 for ppo FEV1% in the pneumonectomy patients (p< 0.01).
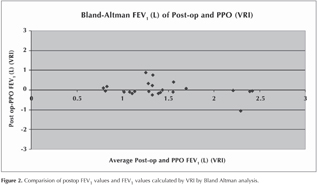
According to the results from Wilcoxon Signed test made for the matched data, no significant difference was found between VRIxp ppo FEV1% and ppo FEV1% values calculated with quantitative perfusion (p> 0.05) (Figure 2).
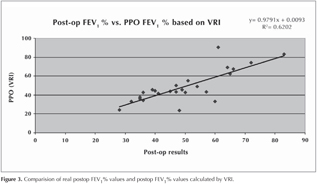
An agreement rate of 52% was found between the ppo values calculated with VRI and with perfusion. This rate was low, although looking to the number of patients having a difference less than 10% between estimated ppo values and actual values at the postoperative 1st month, respective predictive values for ppo VRI and ppo perfusion were found as 84% and 47% (Figure 3).
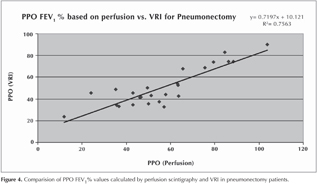
Postop % FEV1 was evaluated between PFTs and VRI with linear regression analysis. The result was statistically significant with a positive correlation (R2= 0.6202) (Figure 4).
Linear regression analysis of ppo FEV1% calculated with VRI and perfusion analysis was statistically significant with a positive correlation (R2=0.7563).
In this study, subgroup analyses could not be carried out because of the small number of the cases underwent pneumonectomy and lobectomy. The linear regression analysis comparing ppo FEV1% values calculated with perfusion scintigraphy and VRI with postoperative FEV1% values calculated with PFTs and VRI was statistically significant with a positive correlation (R2= 0.7563, R2= 0.6202; respectively).
DISCUSSION
In this study, VRI device with which the cancer patients don't expose to radiation and requires no preparation before the procedure was found more significant in prediction postoperative values of the first month than quantitative perfusion scintigraphy with a rate of 47% vs. 84%.
Surgical risks of the lung cancer resection are determined by individual patient characteristics. The protocols estimate these risks from the available data and compare the risks for the patients with sufficient cardiopulmonary reserve in order to predict the relative risk. Therefore, standard surgical resection in the lung cancer should be individually discussed considering the risks and benefits.
In a study by Varela et al., COPD patients were shown to have a better postoperative pulmonary function than estimated by removing of the over-ventilated dysfunctional pulmonary parenchyma (7). However, DLCO measurements may differ in COPD patients due to the uneven ventilation and more reliable test methods must be used (8).
When anatomically operable lung cancer cases are evaluated with spirometry and DLCO, lung perfusion scintigraphy is ordered as a further examination in case of the pulmonary functions determined at the borderline, especially in the patients scheduled for pneumonectomy. Planar-image pulmonary perfusion scintigraphy provides two-dimensional views of the lung, enabling evaluation of postoperative FEV1. Quantitative radionuclide perfusion scanning is used to measure functions of each lung and their segments (9). In general, lung regions affected with cancer have a decreased perfusion, but in some cases increased perfusions have been reported, which underestimate postoperative spirometric parameter percentages (10,11). This is resulted from the overlapping pulmonary regions with different functions. This method has become the most common second line test, because it is simple and available in many hospitals. Algorithm protocols of pulmonary functions should be settled, and new imaging methods should be developed for the patients with pulmonary functions at the borderline.
VRI is a commercially developed acoustic lung imaging system, which was designed for clinical practice, showing the respiratory sound distribution as dynamic grey scale (1). This method may be useful in determining the presence of incidental sounds, although its interpretation requires highly technical expertise and it provides poor information about the spatial distribution of respiratory sounds (12,13). Reproducibility is clinically useful and a prerequisite for development of the acoustical imaging device. Mahagnah and Gavriely demonstrated that the spectral patterns of breathing and backround noise did not show a significant difference between the two recording sessions (14).
Above-mentioned studies which proved that VRI xp device was sufficient in detection of the significant changes in pulmonary sound demonstrated normal pulmonary sound have specific features differentiating them from the abnormal sounds, supporting the potential clinical value of acoustic lung imaging (15,16).
In a study by Jimenez et al., perfusion scintigraphy, VRI, FEV1 and DLCO were measured in 58 patients scheduled for operation due to lung cancer and measurements of FEV1 and DLCO were repeated after 4 and 6 weeks of the surgery. No significant difference was found between the estimated and postoperative FEV1 values (p> 0.05). Average estimated postoperative pulmonary functions were found with 0.74 correlation and 0.70 concordance for the perfusion and VRI (17). In this study, looking to the number of patients having a difference less than 10% between estimated ppo values and actual values at the postoperative 1st month, respective predictive values for ppo VRI and ppo perfusion were found as 84% and 47%. This indicates that significance of ppo VRI was high in prediction of 1st month postoperative values in our patients. It must be noted that VRI does not measure perfusion and ventilation of the lung, and it uses acoustic energy.
No correlation was found between VRI and perfusion in a study by Morice et al., while rate of agreement was found as 52% between ppo VRI and ppo perfusion in the statistical analysis of the first part of our study. This rate was low, although a positive correlation was observed with linear regression analysis on the comparison of ppo FEV% values calculated with VRI and perfusion in 11 patients undergone pneumonectomy (R2= 0.75) (18).
Limitations of this study were as follows; because of the small number of the patients, subgroup analysis could not be made; DLCO was not performed during the study and FEV1 results were analyzed only in the early period.
Although this method is easy to implement and to be learned, following the technical rules and accurate interpretation are crucial. Well isolation of the backround noise and prevention the patients from excessive secretion are necessary for the recording quality. Exclusion criteria must be considered. Care must be taken for reproducibility and technical sufficiency of the records as well as the clinical and radiologic features of the patient should be taken into account during the interpretation. So that in this study, the recording was performed by a technically trained single pulmonologist.
In conclusion, we hope use of VRI device as the second-line test for the preoperative evaluation will increase, because of its being a simple, non-invasive, radiation free, easy to implement at bedside and having a low total cost. We believe that, with further studies, acoustic lung imaging will be added to the non-invasive tools used for preoperative evaluation.
CONFLICT of INTEREST
None declared.
REFERENCES
- Dellinger RP, Parillo JE, Kushnir A, Rossi M, Kushnir I. Dynamic visualization of lung sounds with a vibration response device: a case series. Respiration 2008;75(1):60-72.
- Dellinger RP, Jean S, Cinel I, Tay C, Rajanala S, Glickman YA, et al. Regional distribution of acoustic-based lung vibration as a function of mechanical ventilation mode. Crit Care 2007;11(1):R26.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319-38.
- Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. American College of Chest Physicans Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party Guidelines on the selection of patients with lung cancer for surgery, Thorax 2001;56:89-108.
- Corris PA, Ellis DA, Hawkins T, Gibson GJ. Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax 1987;42:285-291.
- Varela G, Brunelli A, Rocco G, Marasco R, Jimenez MF, Sciarra V, et al. Predicted versus observed FEV1 in the immediate postoperative period after pulmonary lobectomy. Eur J Cardiothorac Surg 2006;30:644-8.
- Horstman MJ, Mertens FW, Schotborg D, Hoogsteden HC, Stam H. Comparison of total-breath and single-breath diffusing capacity in healthy volunteers and COPD patients. Chest 2007;131:237-44.
- Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10.
- Mariano-Goulart D, Barbotte E, Basurko C, Comte F, Rossi M. Accuracy and precision of perfusion lung scintigraphy versus 133Xe-radiospirometry for preoperative pulmonary functional assessment of patients with lung cancer. Eur J Nucl Med Mol Imaging 2006;33:1048-54.
- Chenuel B, Haouzi P, Olivier P, Marie PY, Chalon B, Borrelly J. Effect of exercise on lung-perfusion scanning in patients with bronchogenic carcinoma. Eur Respir J 2002;20:710-6.
- Sovijarvi AR, Helisto P, Malmberg LP, Kallio K, Paajanen E, Saarinen A, et al. A new versatile PC-based lung sound analyzer with automatic crackle analysis (HeLSA); repeatability of spectral parameters and sound amplitude in healthy subjects. Technol Health Care 1998;6:11-22.
- Murphy RL, Vyshedskiy A, Power-Charnitsky VA, Bana DS, Marinelli PM, Wong-Tse A, et al. Automated lung sound analysis in patients with pneumonia. Respir Care 2004;49:1490-7.
- Mahagnah M, Gavriely N. Repeatability of measurements of normal lung sounds. Am J Respir Crit Care Med 1994;149:477-81.
- Kompis M, Pasterkamp H, Wodicka GR. Acoustic imaging of the human chest. Chest 2001;120:1309-21.
- Ploysongsang Y, Pare JA, Macklem PT. Lung sounds in patients with emphysema. Am Rev Respir Dis 1981;124:45-9.
- Jimenez U, Marina N, de Santamaria EL, Pac JJ, Galdiz JB. Evaluation of the utility of vibration response imaging device and Operation Planning Software in the assessment of patients before lung resection surgery. Eur J Cardiothorac Surg 2010;37(5):1185-90.
- Morice RC, Jimenez CA, Eapen GA, Mehran RJ, Keus L, Ost D. Using quantitative breath sound measurements to predict lung function following resection. J Cardiothorac Surg 2010;12;5:81.
Yaz??ma Adresi (Address for Correspondence)
Dr. Nurdan ??M?EK VESKE
?stanbul Yedikule G???s Hastal?klar? ve
G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
G???s Hastal?klar? Klini?i, Zeytinburnu,
?STANBUL - TURKEY
e-mail: nrdnsimsek@gmail.com