RESEARCH ARTICLE
Doi: 10.5578/tt.7717
Tuberk Toraks 2014;62(3):199-206

Akut pulmoner tromboemboli sonras? geli?en kronik tromboembolik pulmoner hipertansiyon s?kl???
?smail KAYAALP1, Yelda VAROL1, P?nar ??MEN1, Fatma DEM?RC? ??SULAR1, Nuran KATGI1, Mehmet ?NL?1,
Cenk KIRAKLI1, Salih Zeki G??L?1, Oktay ERGENE2
1 ?zmir Dr. Suat Seren G???s Hastal?klar? ve Cerrahisi E?itim ve Ara?t?rma Hastanesi, G???s Hastal?klar? B?l?m?,
?zmir, T?rkiye
1 Department of Chest Diseases, Izmir Dr. Suat Seren Chest Diseases and Surgery Training and Research Hospital,
Izmir, Turkey
2 Dokuz Eyl?l ?niversitesi T?p Fak?ltesi, Kardiyoloji Anabilim Dal?, ?zmir, T?rkiye
2 Department of Cardiology, Faculty of Medicine, Dokuz Eylul University, Izmir, Turkey
?ZET
Akut pulmoner tromboemboli sonras? geli?en kronik tromboembolik pulmoner hipertansiyon s?kl???
Giri?: Kronik tromboembolik pulmoner hipertansiyon (KTEPH) ciddi morbidite ve mortaliteye neden olan az?msanmayacak s?kl?kta geli?en, tedavi edilebilir, k?smen de ?nlenebilir bir komplikasyondur. ?al??mam?zda; tan?da ventilasyon/perf?zyon (V/Q) sintigrafisi ile ?ok dedekt?rl? bilgisayarl? tomografi (?DBT) pulmoner anjiyografi gibi noninvaziv tetkikler kullan?larak akut pulmoner tromboemboli (PTE) sonras?nda geli?en KTEPH s?kl???n?n saptanmas? ama?lanm??t?r.
Materyal ve Metod: Ocak 2010 ile Aral?k 2012 tarihleri aras?nda ilk kez pulmoner emboli tan?s? konulan 99 hasta ?al??maya dahil edildi. En az ?? ay antikoag?lan tedavi alm?? hastalara transtorasik ekokardiyografi (TTE) uyguland? (n= 85). TTE'de, SPAP de?eri > 30 mmHg olarak ?l??len ve/veya sa? kalp i?lev bozuklu?u bulgusu saptanan 31 (%34.4) olguya, ?DBT pulmoner anjiyografi ile birlikte V/Q sintigrafisi yap?ld?. ?DBT pulmoner anjiyografide rezid?el kronik tromb?s bulgular? ve/veya V/Q sintigrafisinde segmental perf?zyon defekti ya da defektleri olan olgulara, sa? kalp kateterizasyonu (SKK) yap?ld? (n= 7). Ortalama PAB ?l??ld? ve vazoreaktivite testi yap?ld?. SKK esnas?nda nonkontrast madde pulmoner arterlere verilerek pulmoner arteriyografi g?r?nt?leri al?nd?.
Bulgular: PTE tan?l? hastalar?n 44'? erkek, 55'i kad?nd?. Olgular?n ya? ortalamas? 60 ? 17 ve median ya? 49 (38-67)'du. Olgular?n %63.6's?nda en az bir tane olmak ?zere ek hastal?k anamnezi, %91.9'unda en az bir tane PTE risk fakt?r? mevcuttu. Tan? an?nda 24 olgu masif, 61 olgu submasif ve 14 olgu nonmasif PTE olarak de?erlendirildi. On dokuz (%19.1) olguya trombolitik tedavi uyguland?. Di?er 80 (%80.8) olguya INR de?eri terap?tik aral?kta olacak ?ekilde standart antikoag?lan tedavi verildi. Olgular?n %79.8'inde tromboemboli yayg?nl??? bilateral, %21.8'inde unilateraldi. Minumum 1 maksimum 2 y?l takip sonras?nda 5 (%5.5) hasta KTEPH tan?s? ald?. KTEPH gelimi ile ya?, PTE etiyolojik risk fakt?r?, trombolitik tedavi almak, PTE tipi ve yayg?nl??? aras?nda univaryan analizde istatistiksel anlaml?l?k saptanmad?.
Sonu?: PTE'nin ?nlenebilir bir komplikasyonu olan KTEPH takip edilen hastalarda olduk?a y?ksek bir insidansta saptanm??t?r.
Anahtar kelimeler: Pulmoner emboli, kronik tromboembolik pulmoner hipertansiyon, g?r?nt?leme y?ntemleri
SUMMARY
The incidence of chronic thromboembolic pulmonary hypertension secondary to acute pulmonary thromboembolism
Introduction: Chronic thromboembolic pulmonary hypertension (CTEPH) is a curable and partially preventable complication, with a substantial incidence, leading to severe morbidity and mortality. The aim of the present study was to find out the incidence of CTEPH secondary to acute pulmonary thromboembolism (PTE) using non-invasive procedures such as ventilation/perfusion (V/Q) scintigraphy and pulmonary multidetector CT (MDCT) angiography in determining the diagnosis of CTEPH.
Materials and Methods: The study included a total of 99 patients diagnosed with initial PTE between January 2010 and December 2012. The patients who received anticoagulant therapy at least for three months underwent transthoracic echocardiography (TTE) (n= 85). Thirty one patients with a SPAP value > 30 mmHg and/or an evidence of right ventricular dysfunction in TTE underwent MDCT pulmonary angiography and V/Q scintigraphy. The patients with an evidence of residual chronic thromboembolic signs in MDCT pulmonary angiography and/or segmental perfusion defect(s) in V/Q scintigraphy underwent right heart catheterization (RHC) (n= 7). The mean PAP was measured, and a vasoreactivity test was performed. During RHC, a non-contrast medium was delivered to the pulmonary arteries for pulmonary arteriography imaging.
Results: Among patients diagnosed with PTE, 44 were male and 55 were female. The mean age was 60 ? 17 years. Of these patients, 63.6% had history of at least one additional disease and at least one risk factor for PTE. During diagnosis, 24 subjects were considered having massive, 61 submassive and 14 non-massive PTE. Nineteen (19.1%) patients received thrombolythic therapy. Other 80 (80.8%) patients received standard anticoagulant therapy with an INR value within the therapeutic range. In 79.8% of patients, thromboembolism was bilateral, and it was unilateral in 21.8%. After a minimum of 1 year, and maximum of 2 years follow up five subjects (5.5%) were diagnosed with CTEPH. The univariate analysis showed no association between the development of CTEPH and factors like; age, etiologic risk factors for PTE, receiving thrombolytic treatment, prevalence and type of PTE.
Conclusion: Potentially preventabl complication of pulmonary embolism; CTEPH, had a substantial incidence during follow-up.
Key words: Pulmonary embolism, chronic thromboembolic pulmonary hypertension, imaging techniques
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is a serious condition characterized by intraluminal thrombus organization and fibrous stenosis or complete obliteration of the pulmonary arteries. CTEPH is commonly seen as a long-term complication of acute pulmonary embolism (PE) (1). The incidence of CTEPH has been reported by different studies to be between 0.1% and 8.8% in patients after acute PE (2-4). The diagnosis of CTEPH is often delayed since the symptoms are non-specific (5-7).
The first step towards making the correct diagnosis is the assessment of regional lung perfusion defects, which is commonly carried out by scintigraphic planar lung scans. Planar perfusion scintigraphy is useful for initial evaluation of CTEPH (5-7).
Contrast-enhanced CT pulmonary angiography (CTPA) is also commonly used for evaluation of acute PE and CTEPH. Over the past few years, CT scanning has proven its diagnostic value in cases of acute pulmonary embolism (9-11). It has also been proposed as a reliable and less invasive tool for the diagnosis of CTEPH (12). Because CTEPH is a relatively common, life-threatening complication of pulmonary embolism, diagnostic and therapeutic strategies for early detection and prevention of CTEPH are needed. Based on this, the aim of our study was to determine the incidence of CTEPH after acute PTE with ventilation/perfusion (V/Q) scintigraphy and/or with pulmonary multidetector CT (MDCT) angiography.
MATERIALS AND METHODS
The present prospective study included patients who were diagnosed with PTE between January 2010 and December 2012, prescribed with an outpatient treatment plan and who received anticoagulant therapy at least for 3 months. Those patients who were diagnosed between January 2010 and January 2012 were followed for a minimum of 1 year, and maximum of 2 years. We examined type of PTE at diagnosis, prevalence and treatment regimen, age, sex, medical history of concomittant diseases, the stage according to the New York Heart Association (NYHA) Functional Classification and etiologic risk factor(s).
A Doppler ultrasonography exam of the bilateral lower extremity venous system was performed using the General Electric Logiq 5 Pro Doppler machine. The exam covered the bilateral inguinal area from the main femoral vein to the level of cruris vein using a linear transducer with a variable frequency, ranging from 5 to 10 megahertz. During the examination, patency of both main femoral, superficial femoral, deep femoral, popliteal and saphenousveins was evaluated by a color Doppler based on the compression of vein in response to the transducer. The flow in cruris veins was evaluated by a maneuver applied on the gastrocnemius muscle.
Each patient underwent a control TTE examination, which was performed by expert cardiologist sapproximately at the end of one year follow up. In TTE, patients with a SPAP ≤ 30 mmHg and without any evidence of right heart failure and chronic residual thrombus by MDCT pulmonary angiography were considered negative prior to any further examination. In TTE, 31 patients with a SPAP > 30 mmHg and/or any evidence of right heart failure (34.4%) underwent V/Q scintigraphy along with MDCT pulmonary angiography.
MDCT pulmonary angiography examinations were performed with a 16 slice-Philips MX and Philips Brilliance-6 multidetector row scanner.? Intravenous contrast agent was administered using the Mallinckrodt and Optistar BT automatic injector systems, respectively. A guiding image was obtained with the patient in supine position to determine the area of study from the apex to the diaphram. During imaging, a 60-90 mL (1 mL/kg) non-ionic contrast agent (370 mg/mL) was delivered via an automatic injector through the vascular access established by a 20 gauge cannula placed into an antecubital vein at a rate of 3.5 mL/sec. A reference image was obtained at the level of the main pulmonary artery by a 16- slice Philips MXscanner (thickness of section; 5 mm, interslice distance; 2.5 mm; threshold; 80, collimation; 16 x 1.5, pitch; 0.9, rotation time; 0.75 sec and FOV; wide) and cross sectional images were obtained using predefined parameters at 250 mA/sec. During imaging with Philips Brilliance- 6 scanner,cross sectional images were obtained with a delayed time of 12 to 16 secs from the start of injection depending on the cardiac performance status of the patient (thickness of section: 5 mm; interslice distance: 2.5 mm; collimation: 6 x 3; pitch: 0.9; rotation time: 0.75 sec; FOV: wide) using predefined parameters at 220 mA/sec. All MDCT pulmonary angiography examinations were performed in the craniocaudal direction in a single breath-hold period.
Pulmonary perfusion scintigraphy was performed witha Philips Brightview dual-head gamma camera following intravenous administration of Tc-99m macroaggregated albumin based on the principle of radiopharmaceutical's distribution within the lung alveoli/capillaries in parallel to the distribution of pulmonary arteries. Injection was made during deep and regular breathing in lying position. The imaging included anterior, posterior, left lateral, right lateral, left posterior oblique, right posterior oblique, left anterior oblique, and right anterior oblique positions.
Pulmonary ventilation scintigraphy was also performed with a Philips Brightview dual-head gamma camera. The patients underwent imaging after inhalation ofa mixture of Tc-99m diethylene triamine penta acetic acid with 3-4 L/min O2 in a ventilation apparatus for 10 minutes. The imaging included anterior, posterior, left lateral, right lateral, left posterior oblique, right posterior oblique, left anterior oblique, and right anterior oblique positions. All V/Q scintigraphies were evaluated based on the modified PIOPED criteria (13,14).
Both examinations excluded the patients with no evidence of chronic residual thrombus and/or segmental perfusion defect for CTEPH. Any patient with an evidence of chronic residual thrombus during MDCT pulmonary angiography and/or segmental perfusion defect(s) during V/Q scintigraphy underwent right heart catheterization using a Siemens Artis Zee DSA system. Pulmonary artery was accessed through the femoral vein with a 6 French catheter. The mean PAP was measured, and a vasoreactivity test was performed. During RHC, a non-contrast medium was delivered to the pulmonary arteries for pulmonary arteriography imaging.
For evaluations, we used Statistical Package for the Social Sciences Program (SPSS 17.0 for Windows, SPSS Inc., Chicago, Il., USA). Contiunous variables and all sub-groups were tested for normal distribution. A correlation coefficient was calculated to evaluate the relationship between numeric variables. For all tests, the error rate was alpha 5%, with a two-tailed test. If the p value was less than 0.05, the difference between the groups was considered statistically significant.
RESULTS
Among patients with PTE, 44 were male, and 55 were female. The mean age was 60 ? 17 years. Of these patients, 63.6% had at least one additional disease.The major risk factor for most of the patients was advanced age (55.5%), followed by immobilization and surgical intervention, respectively. There was at least one risk factor of PTE in 91.9% of our patients (Table 1).
Nine patients died during the follow-up.
During diagnosis, 24 subjects were considered having massive, 61 submassive and 14 non-massive PTE. Nineteen (19.1%) patients received thrombolythic therapy. Other 80 (80.8%) patients received standard anticoagulant therapy with an INR value within the therapeutic range. In 79.8% of patients, thromboembolism was bilateral, and it was unilateral in 21.8%. Thirty four (34.3%) patients had TTE during the diagnosis and the median estimated SPAP was 49 mmHg (range 38-67).
Fifty one (51.1%) patients underwent Doppler ultrasonography examination of the bilateral lower extremity venous system during diagnosis. Thirty four patients had deep vein thrombosis.
The median time to evaluation for CTEPH in patients who received effective anticoagulant therapy at least for 3 months and who were still on anti-cogaulant therapy was 6 months (range 4-9). The median SPAP was 32 mmHg (range 27-38) in all alive patients during the control examination for TTE. There were 5 patients who could not come to the TTE examination and these patients was selected as dropped patients. Patients who were evaluated for CTEPH were classified according to the NYHA Functional Classification (Table 2).
Eighty five patients who had an acute PTE episode underwent control MDCT pulmonary angiography for CTEPH while 31 patients who had an estimated SPAP > 30 mmHg and/or evidence of right heart disease underwent V/Q scintigraphy along with a MDCT pulmonary angiography. The V/Q scintigraphy results showed high probability in 2 (6.4%) patients , moderate probability in 2 (6.4%) patients, low probability in 7 (22.5%) patients, and normal in 20 (64.5%) patients according to the modified PIOPED criteria.
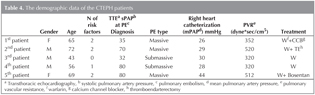
MDCT pulmonary angiography of five patients showed findings consistent with chronic thromboembolism. The findings, the most common being widening of the main pulmonary arteries, included total obstruction in pulmonary arteries, peripheral scar tissue, thinning of pulmonary arteries and partial obstruction, respectively (Table 3).
Seven patients underwent right heart catheterization to confirm the diagnosis. The median PAP was 29 mmHg (range 28-34). During RHC, pulmonary DSA was performed in 5 patients, who all showed defects with the contrast dye, consistent with chronic thromboembolism. Five patients were diagnosed with CTEPH (Table 4). The incidence of CTEPH was 5.5%. The univariate analysis showed no association between the development of CTEPH and factors like; age, etiologic risk factors for PTE, receiving thrombolytic treatment, prevalence and type of PTE.
DISCUSSION
Pulmonary embolism is a clinical condition where pulmonary arteries and/or their branches become occluded by various substances (such as fat particles, foreign bodies, tumor cells), mostly by thrombotic particles getting into the circulation from the thrombi in deep veins of the lower extremity. Quite a number of risk factors have been described by studies on development of PTE, which is a common disease, challenging in diagnosis with a high rate of mortality and morbidity (1,7). Similar to many other studies, in our study the most common risk factors were advanced age, immobilization and surgical intervention respectively.
Although previously CTEPH was known to be a rare complication of PTE, recent studies have shown that the incidence of symptomatic and/or asymptomatic CTEPH is more than previously thought (15,16). Several reports emphasize that attention should be paid to young patients with idiopathic PTE, frequent PTE episodes and larger perfusion defects who may develop CTEPH. In a study by Pengo et al. who evaluated the potential risk factors for CTEPH, a significant association was found with multiple PTE episodes, a larger perfusion defect at the time of diagnosis, younger age and idiopathic presentation of PTE (16). In this study while the univariate analysis showed a significant relationship in those who had thrombolytic treatment, there was no significant relationship in the multivariate analysis between the two arms. It was mainly attributed to the use of thrombolytic treatment mostly in massive PTE. Since there was an increased risk associated with recurrent PTE in those patients with no regular treatment, they claimed that it might be attributed to inadequate treatment. In our study the univariate analysis showed no association between the development of CTEPH and factors like; age, etiologic risk factors for PTE, receiving thrombolytic treatment, prevalence and type of PTE. We believe it might be related with the small numbers of patients with CTEPH we had detected.
In previous studies, the incidence of CTEPH has been reported to be between 0.1% and 3.8% (5-17). Becattini et al. studied 259 patients with PTE to determine the incidence of CTEPH secondary to the initial episode of PTE (18). Each patient received anticogulant therapy, at least for 3 months, and at most for 1 year, with a follow up every three months during the first year, and then every 6 months for the next 3 years. All patients who described persistent dyspnea either at rest or on exertion underwent TTE, during which any patient with a SPAP over 30 mmHg further underwent V/Q scintigraphy and pulmonary arteriography. The patients with a mean PAP ≥ 25 mmHg, normal PKB and arteriographic thromboembolism were considered to have CTEPH (18). In this study including only symptomatic subjects, the incidence of CTEPH was 1%. The INR was within the therapeutic range in more than 75% of the patients, reporting that the incidence associated with their careful anticoagulant therapy was low. Frederikus et al. found pulmonary hypertension in 19 of 866 patients with acute PTE after a follow up of 34 months (19). Four of these 4 (0.57%) patients were diagnosed with CTEPH. In a study by Korkmaz et al. carried out in Turkey, 325 patients with a diagnosis of PTE were followed for an average of 16.3 months (6-50.7 months) (20). Data on recurrence, residual thrombi, mortality and CTEPH were collected. The incidence of CTEPH was 4.6%. The incidence of residual chronic thrombus was 48% at month 3 after the first episode, 27.4% at month 6, and 18.2% at month 12, and they highlighted the significance of close monitoring of patients with PTE for an early diagnosis of CTEPH.
A study carried out by Mi et al. in China found an incidence of 14.4% for CTEPH (21). Similarly the report published by Pepke Zaba in 2010 indicated that the incidence of CTEPH was between 1.3% and 5.1% after one year of PTE treatment, and between 0.8% and 3.8% after 2 years (22). Dentali et al. also evaluated 91 patients with PTE by V/Q scintigraphy and TTE (23). CTEPH was defined as a SPAP > 40 mmHg and presence of a residual perfusion defect in V/Q scintigraphy. All patients were evaluated by TTE at months 6 and 12 after the PTE episode, with CTEPH in 8 patients (with an incidence of 8.7%). Using a questionnaire on symptoms, 4 patients were found to have asymptomatic CTEPH, and 4 other patients symptomatic CTEPH. In our study, the incidence of CTEPH was 5.5%. Two of our patients were asymptomatic. The incidence of CTEPH in our study was comparable to those studies which also included asymptomatic patients. However, the rate was higher when all studies were considered. An analysis of the records showed that our patients had some of their INR follow-up done in hospitals close to their houses as we are a regional hospital. It is difficult for us to affirm whether they received anticoagulant therapy within the therapeutic range since we are unable to have access to their complete INR follow-up. Studies demonstrated that the proportion of patients with INR values below therapeutic range was 20% despite regular INR monitorization (16).
An overview of studies on CTEPH carried out up to date shows that it has a substantial incidence following acute PTE, while recent studies indicate increasing rates. Therefore, we need easily applicable, easily accessible, and less invasive diagnostic tests, with high sensitivity and specificity in order to detect patients accurately and on time. Nistal and Martin define imaging tests as cornerstone of the diagnosis of CTEPH (24). V/Q scintigraphy, TTE, MDCT pulmonary angiography, magnetic resonance imaging pulmonary angiography and pulmonary DSA are unquestionably very convenient imaging methods. However, they cannot be used routinely in all patients. Use of TTE and V/Q scintigraphy is common to exclude CTEPH, which develops secondary to acute PTE. When pulmonary hypertension is suspected, TTE represents the most common initial examination. Detection of tricuspid regurgitation jet and measurement of the maximal jet velocity by Doppler help estimate the SPAP value. The sensitivity and specificity of this method for detection of SPAP are 79-100% and 60-98%, respectively (25). In a study by Boilson et al. with 1000 CTEPH patients who underwent PEA operation, the pre-operative TTE and right atrial and pulmonary artery pressures as measured by RHC were correlated (p < 0.0001) (26). In recent studies it is indicated that the diagnosis of CTEPH can be excluded in patients with unexplained history of PTE or pulmonary hypertension if no perfusion defect is found with V/Q scintigraphy and/or when V/Q scintigraphy shows a normal perfusion (22-27). A literature review showed that moderate and high probability V/Q scintigraphy had a high sensitivity and specificity in the diagnosis of CTEPH (22-28). In a study by Fang et al. of 78 patients with pulmonary hypertension, but no history of congenital heart disease and acute PTE, the sensitivity, specificity and accuracy of a high probability V/Q scintigraphy in the diagnosis of CTEPH were 96.0%, 81.1%, and 86.9%, respectively (28).
MDCT pulmonary angiography is an effective method which determines localization and extent of chronic thrombus, and features of central and peripheral arterial thrombi. It may also provide additional information on collateral circulations, and accompanying parenchymal abnormalities. MDCT pulmonary angiography is well tolerated by patients, being a non-invasive method used both in follow up of the disease and evaluation of postoperative outcomes. The sensitivity of MDCT pulmonary angiography is unstable in the diagnosis of CTEPH. In a study by Pitton et al. who made a comparison with pulmonary DSA, the sensitivity of MDCT pulmonary angiography was 70.4% for segmental branches, and 63.6% for subsegmental branches (29).
In a pivotal study by Soler et al. the SPECT perfusion scintigraphy, which is more sensitive than planar perfusion scintigraphy in detecting the pulmonary perfusion defects, was compared with MDCT pulmonary angiography. SPECT perfusion scintigraphy and MDCT pulmonary angiography were performed in 12 patients with CTEPH who were scheduled for a PEA operation, and then they were compared, considering the pulmonary arteriography as the golden standard. They described 140 obstructed, and 40 unobstructed lung segments. SPECT perfusion scintigraphy identified 62% (87/140) of the obstructed and 72% (29/40) of the unobstructed segments whereas MDCT pulmonary angiography identified 47.8% (67/140) of the obstructed, and 80% (32/40) of the unobstructed segments. They indicated that sensitivity for detecting obstructed segments was significantly higher for SPECT perfusion scintigraphy compared to MDCT pulmonary angiography while MDCT pulmonary angiography was superior in detecting unobstructed segments (30).
The diagnostic algorithm used in our study is consistent with current publications. Not all patients underwent RHC and pulmonary DSA. They were used to confirm the diagnosis of CTEPH in patients with an evidence of residual chronic thrombus by MDCT pulmonary angiography, and patients with an evidence of segmental perfusion defect or defects by V/Q scintigraphy. Limited accessibility to the procedure, being risky, although low, and development of other diagnostic methods resulted in its reduced use. Pulmonary DSA is recently indicated for patients who are surgical candidates for a PEA operation, and it is not used for routine diagnosis.
In conclusion, we found a substantial number of patients with pulmonary embolism, who developed CTEPH during follow-up. Further studies are needed to determine preventable causes of a serious complication like CTEPH, reduce its incidence, and develop non-invasive diagnostic methods.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Klok FA, Kralingen KW, Van Dijk APJ, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010;95:970-5.
- Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2001;345:1465-72.
- Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64.
- Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine 2006;85:253-62.
- Auger WR, Kim NH, Trow TK. Chronic thromboembolic pulmonary hypertension. Clin Chest Med 2010;31:741-58.
- Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011;183:1605-13.
- Saltzman HA, Alavi A, Greenpan RH. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 1990;263:2753-9.
- Friera A, Olivera MJ, Su?rez C, Ruiz-Gim?nez N, Caballero P. Clinical validity of negative helical computed tomography for clinical suspicion of pulmonary embolism. Respiration 2004;71:30-6.
- Schoepf UJ, Goldhaber SZ, Costello P. Spiral computed tomography for acute pulmonary embolism. Circulation 2004;109:2160-7.
- Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology 2004;230:329-37.
- Fedullo PF, Tapson VF. Clinical practice: the evaluation of suspected pulmonary embolism. N Engl J Med 2003;349:1247-56.
- King MA, Ysrael M, Bergin CJ. Chronic thromboembolic pulmonary hypertension: CT findings. AJR Am J Roentgenol 1998;170:955-60.
- Freitas JE, Sarosi MG, Nagle CC, Yeomans ME, Freitas AE, Juni JE. Modified PIOPED criteria used in clinical practice. J Nucl Med 1995;36:1573-8.
- Freeman LM, Krynyckyi BR, Zuckier LS. Enhanced lung scan diagnosis of pulmonary embolism with the use of ancillary scintigraphic findings and clinical correlation. Semin Nucl Med 2001;31:143-57.
- Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151-8.
- Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. ?ncidence of chronic thromboembolik pulmonary hipertension after pulmonary embolism. N Engl J Med 2004;350:2257-64.
- Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: from acute to chronic pulmonary embolism. Proc Am Thorac Soc 2006;3:564-7.
- Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Tailani MR, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary Embolism. Chest 2006;130:172-5.
- Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Viliegen HV, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010;95:970-5.
- Korkmaz A, Ozlu T, Ozsu S, Kazaz Z, Bulbul Y. Longterm outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost 2012;18:281-8.
- Mi J, Sun ZH, Zhong MH, Yang YH, Chan T, Xiong GJ, et al. Predictive factors of chronic thromboembolic pulmonary hypertension in patients with acute pulmonary thromboembolism. Zhonghua Xin Xue Guan Bing Za Zhi 2012;40:497a(abstract).
- Pepke Zaba J. Diagnostic testing to guide the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2010;19:55-8.
- Dentali F, Donadini M, Gianni M, Bertollini A, Squizzato A, Venco A, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009;124:256-8.
- Nistal MA, Martin MT. Imaging tests in chronic thromboembolic pulmonary hypertension. Arch Bronconeumol 2009;45:21-9.
- Trow TK, McArdle JR. Diagnosis of pulmonary arterial hypertension. Clin Chest Med 2007;28:59-73.
- Boilson BA, Pislaru SV, Mc Gregor CG. Accuracy of echocardiographic assessment of pulmonary hypertension severity and right ventricular dysfunction in patients with chronic thromboembolic pulmonary hypertension. Minerva Cardioangiol 2012;60:257-65.
- Hoeper MM, Barber? JA, Channick RN, Hassoun PM, Lang IM, Manes A, et al. Diagnosis, assessment, and treatment of non pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009;30:85-96.
- Fang W, Ni XH, He JG, Liu ZH, Xiong CM, He ZX. Value of radionuclide lung scintigraphy in the diagnosis and quantitative analysis of chronic thromboembolic pulmonary hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2008;36:7a(abstract).
- Pitton MB, Kemmerich G, Herber S, Schweden F, Mayer E, Thelen M. Chronic thromboembolic pulmonary hypertension: diagnostic impact of multislice CT and selective pulmonary DSA. Rofo 2002;174:474-9.
- Soler X, Kerr KM, Marsh JJ, Renner JW, Hoh CK, Test VJ, et al. Pilot study comparing SPECT perfusion scintigraphy with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Respirology 2012;17:180-4.
Yaz??ma Adresi (Address for Correspondence)
Dr. Yelda VAROL
?zmir Dr. Suat Seren G???s Hastal?klar? ve
Cerrahisi E?itim ve Ara?t?rma Hastanesi,
G???s Hastal?klar? B?l?m?,?
?ZM?R - TURKEY
e-mail: yeldavatansever@hotmail.com