RESEARCH ARTICLE
Doi: 10.5578/tt.7902
Tuberk Toraks 2014;62(3):191-198

Akci?er kanserli olgular?n biyolojik ?rneklerinde arsenik ve? kadmiyum d?zeylerinin de?erlendirilmesi
Nalan DEM?R1, Vugar Ali T?RKSOY2, Zeliha KAYAALTI2, T?lin S?YLEMEZO?LU2, ?smail SAVA?1
1 Ankara ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Ankara, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Ankara University, Ankara, Turkey
2 Ankara ?niversitesi T?p Fak?ltesi, Adli T?p Enstit?s?, Ankara, T?rkiye
2 Institute of Forensic Sciences, Faculty of Medicine, Ankara University, Ankara, Turkey
?ZET
Akci?er kanserli olgular?n biyolojik ?rneklerinde arsenik ve? kadmiyum d?zeylerinin de?erlendirilmesi
Giri?: Kronik toksik metal maruziyeti akci?er kanseri nedenleri aras?nda sigaran?n yan? s?ra ?nemli bir role sahiptir. Bu ?al??man?n amac?; biyolojik ?rneklerdeki arsenik ve kadmiyum d?zeyleriyle akci?er kanserinin histopatolojik tipleri aras?ndaki ili?kiyi de?erlendirmektir.
Materyal ve Metod: Akci?er kanseri tan?s? alan 72 olguda prospektif olarak yap?lan bu ?al??ma 2009-2013 tarihleri aras?nda tek bir merkezde ger?ekle?tirildi. Olgular?n tedavi ?ncesinde al?nan biyolojik ?rneklerinde (kan, sa?, idrar) arsenik ve kadmiyum d?zeyleri atomik absorpsiyon spektrometriyle ?l??ld?. Akci?er kanserli olgular?n ?zellikleri ve metal d?zeyleri istatistiksel olarak kar??la?t?r?ld? (Bu ?al??man?n g?c?: 0.74 idi).
Bulgular: Yetmi? iki olgunun (7 K/65 E, ort. ya?: 62.19? ? 8.74 y?l), 56 (%77.8)'s? k???k h?creli d??? akci?er kanseri (KHDAK), 16's? k???k h?creli akci?er kanseri (KHAK) idi. TNM evrelemesine g?re, KHDAK'lar?n 27'si evre IV, KHAK'l? olgular?n 14'? yayg?n hastal?kt?. Olgular?n kan, sa? ve idrar ?rneklerinde, ortalama arsenik d?zeyleri s?ras?yla 23.1 ? 9.2 ?g/L, 0.6 ? 0.3 ?g/g ve 3.6 ? 1.9 ?g/L iken; kadmiyum d?zeyleri 1.2 ? 0.8 ?g/L, 0.3 ? 0.1 ?g/L ve 2.8 ? 1.6 ?g/L idi. Kan ile idrar arsenik d?zeyleri aras?nda istatistiksel olarak anlaml? negatif korelasyon (r= -0.350; p= 0.025), kan ile sa? kadmiyum d?zeyleri aras?nda ise pozitif korelasyon saptand? (r= -0.371; p= 0.017). Her iki metal d?zeyi de (idrar arseni?i hari?) KHDAK'l? olgularda; KHAK'a g?re daha y?ksekti, ancak istatistiksel olarak anlaml? de?ildi. TNM evrelemesi ve mortalite a??s?ndan anlaml? bir ili?ki bulunmad? (p> 0.05).
Sonu?: Bu ?al??mada, biyolojik ?rneklerdeki arsenik ve kadmiyum d?zeyleriyle akci?er kanseri tipi, evrelemesi ve mortalite aras?nda anlaml? bir ili?ki saptanmad?. Daha ileri ?al??malara ihtiya? oldu?u d???n?ld?.
Anahtar kelimeler: Akci?er kanseri, arsenik, kadmiyum, kan, sa?, idrar
SUMMARY
The evaluation of arsenic and cadmium levels in biological samples of cases with lung cancer
Introduction: Chronic exposure to the toxic metals plays an important role among the causes of lung cancer beside of smoking. We aimed to evaluate the association between the histopathologic type of lung cancer and arsenic and cadmium levels in biological samples.
Materials and Methods: This study in a single center was conducted through the years 2009-2013, including 72 patients with lung cancer, within a prospective study design. Biological samples (whole blood, scalp hair, urine) of subjects obtained before the treatment, and arsenic and cadmium levels were analyzed by atomic absorption spectrophotometer. The characteristics of lung cancer cases and metal levels were compared statistically (power: 0.74).
Results: Fifty six (77.8%) of patients were non-small cell lung cancer (NSCLC), 16 (22.2%) were small cell lung cancer (SCLC) in 72 study subjects (7 F/65 M, mean age= 62.2 ? 8.7 years). According to TNM staging, 27 of NSCLC were stage IV, 14 of SCLC were extensive disease. In blood, scalp hair and urine samples of cases, mean arsenic levels were 23.1 ? 9.2 ?g/L, 0.6 ? 0.3 ?g/g and 3.6 ? 1.9 ?g/L, while cadmium levels were 1.2 ? 0.8 ?g/L, 0.3 ? 0.1 ?g/L and 2.8 ? 1.6 ?g/L, respectively. A significant negative correlation was found between blood and urine arsenic levels (r= -0.350; p= 0.025). Blood and hair cadmium levels were also significant positive correlated (r= -0.371; p= 0.017). Both of metal levels except of urine arsenic were higher in NSCLC patients than SCLC, without any statistical significance. No significance relation was found in terms of TNM staging and mortality (p> 0.05).
Conclusion: Any difference was observed between the arsenic and cadmium levels measured in biological samples and histopathological type, staging and mortality of patients with lung cancer in this study. We thought that further studies are needed.
Key words: Lung cancer, arsenic, cadmium, blood, scalp hair, urine
INTRODUCTION
Although smoking is the most common cause of lung cancer, chronic exposure to toxic metals from environment or cigarette smoke may also play role in the development of lung cancer due to possible mechanisms such as oxidative stress, DNA repair modulation and disturbances of signal transduction pathways (1).
Arsenic and cadmium are toxic metals which have classified as group 1 human carcinogens by the International Agency for Research on Cancer (IARC)(2,3). These metals occur naturally in earth's crust with higher concentrations and the most important sources of them for general population are drinking water, food, air pollution and cigarette smoke. Chronic exposure to these metals has been reported worldwide affecting nearly one hundred million people in most countries (4). Previous studies have shown that occupational exposure to arsenic and cadmium and living in the polluted environment are associated with lung cancer (5). However, inadequate data present on the relationship between lung cancer and environmental low-dose arsenic and cadmium exposure and it should keep in mind among the lung cancer causes.
Arsenic and cadmium can be accumulated in liver, kidney, lung and other tissues because of their long biologic half-life achieving from 17 to 33 years. Additionally, they can also be determined in hair, nail, skin and other biological samples (2,6).
The development of lung cancer and the other internal cancers due to chronic exposure to arsenic and cadmium have been well defined since 1950, in addition to their non-neoplastic effects (6). Previous studies have identified associations between levels of these metals in urine, blood and hair with lung cancer (7,9). However, there are few studies investigating the metal levels of lung cancer patients from Turkish population, to date. The objectives of this study were to determine the levels of arsenic and cadmium in biological samples obtaining from blood, scalp hair and urine whom patients diagnosed lung cancer, and to evaluate association of these metals and histopathologic type of lung cancer in a single center.
MATERIALS AND METHODS
This study was approved by the local ethics committee in accordance with institutional rules and Declaration of Helsinki requirements.
Subjects
Totally, 72 patients with lung cancer who admitted to our clinic and followed-up between the January 2009-February 2013, were investigated in a prospective study. The diagnosis of lung cancer was confirmed histopathologically for each patient.
Samples Collection and Storage
Whole blood, scalp hair and urine samples obtaining before the treatment were collected from each patients, following informed written consent. Venous blood samples (10 mL) were collected by using metal-free heparinized vacutainer tubes (Becton Dickinson, Rutherford, NJ, USA). The scalp hair samples were taken from occipital part of the scalp were washed and treated as reported in previous studies. After washing scalp hair samples were dried in electrical oven at approximately 75?C and stored pre-cleaned plastic bags with identify numbers. The urine samples were also collected in the morning from each patient (10-12). All of the samples prevented of the metal contamination, put into sterile vials and immediately stored at -20?C for elemental analysis.
Metal Analysis
The analysis of biological samples for arsenic and cadmium was performed at the Institute of Forensic Sciences of Ankara University, Toxicology Laboratory in this study.
The concentrations of elements were measured by means of an atomic absorption spectrophotometer after microwave-assisted acid digestion. The validity and accuracy was checked by conventional wet acid digestion method and using certified reference materials. The overall recoveries of all elements were found in the range of 98.1-99.4% of certified values (13,14).
The lineer range of calibration curve reached from the detection limit up to 10 and 10 for arsenic and cadmium, respectively. The detection limit (LOD) was defined as 3 s/m, where ?s' is the standard deviation corresponding to 10 blank injections and ?m' is the slope of calibration graph. The LOD of 0.034 and 0.005 were calculated for arsenic and cadmium, respectively. The validity and efficiency of the microwave assisted digestion method on same certified reference material as reported in previous work (10,11).
Study Design
The demographic data, detailed history, clinical and laboratory findings of all patients were recorded on admission to our institution. According to the smoking habits, subjects were classified into the following subgroups: currents smokers, ex-smokers and non-smokers. Current smokers included even subjects who stopped tobacco smoking from no more than one year; whereas the non-smokers group included both subjects who had never smoked and ex-smokers from at least 15 years who smoked no more than 30 pack years (PY).
Each patient was screened for lymph node involvement and distant metastasis by one or more diagnostic method including thorax-abdomen computed tomography (CT), whole body bone scintigraphy and positron emission tomography (PET) before the treatment and they were classified according to TNM (tumor node metastasis) staging for lung cancer (15).
None of the enrolled patients received previous chemotherapy or radiotherapy treatments before the collecting samples.
Follow Up
Patients were followed up to February 2013 for four years. Mortality rate was recorded in all study subjects.
Statistical Analysis
Data were assessed by using SPSS 16.0 for Windows software package. Student t and one-way ANOVA parametric tests were used for the comparison of groups in terms of metric variables and data were measured as mean ? standart deviation. The chi-square test was used to compare categorical variables. In addition, case summary reports and frequency charts were used to analyze the group variables. All p values were two-tailed and a p value 95% (p< 0.05) and 99% (p< 0.01) were considered statistically significant.
RESULTS
Sixty five male and 7 female, totally 72 patients ranged from 46-85 years old were enrolled in the study. Mean age of all cases was 62.2 ? 8.7 years. On admission, 50 (69.4%) patients were current smoker, 9 of patients were ex-smoker with mean pack year was 52.4 ? 23.7. Only 13 patients were non-smoker. The history of occupational possible exposure to toxic gas and inhalation was obtained in 7 (9.7%) patients, but none of these had arsenic and cadmium exposure due to their work, directly. Six cases had biomass exposure, while 16 cases had as best exposure in their life. Nine (12.5%) patients had past records of cancer in family members. Two (2.8%) of all? were history of previous cancer which developed from another organ. Among 47 (65.3%) patients, the most common existing co-morbidities were chronic obstructive pulmonary disease (n= 20, 27.8%) and atherosclerotic cardiac diseases (n= 18, 25.0%) (Table 1).
On the evaluation of thorax CT, tumor diameters of all patients were varied 1.5 to 11 cm. The most common localization of tumor on thorax CT was right hilar region (n= 18, 25.0%). The diagnoses of patients were confirmed by the samples obtained from fiberoptic bronchoscopy (n= 44, 61.1%), surgical resection material (n= 14, 19.4%), and transthoracic needle biopsy (n= 14, 19.4%).
The histopathological diagnoses were non-small cell lung cancer (NSCLC) (n= 56, 77.8%) and small cell lung carcinoma (SCLC) (n= 16, 22.2%). In NSCLC group, the subtypes of lung cancer were squamous cell (n= 24, 33.3%), adenocarcinoma (n= 22, 30.6%); unclassified NSCLC (n= 8, 11.1%), and large cell (n= 2, 2.8%).
When we evaluated to the patients according to the TNM staging, while the most common stage was stage IV (n= 27, 37.5%) in NSCLC, it was extensive disease (n= 14, 19.4%) in SCLC group. While 18 (25.0%) cases were treated surgically with and without neoadjuvan/adjuvant chemotherapy and/or radiotherapy, 43 of all cases (59.72%) were treated with chemotherapy and/or radiotherapy. In 9 (12.5%) cases, palliative treatment were administered, and 2 (2.8%) cases? did not accept the treatment (Table 2).
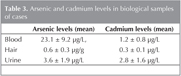
Mean arsenic levels were measured as 23.1 ? 9.2 ?g/L, 0.6 ? 0.3 ?g/g and 3.6 ? 1.9 ?g/L in blood, hair and urine samples, respectively. Mean cadmium levels were also found as 1.2 ? 0.8 ?g/L, 0.3 ? 0.1 ?g/L and 2.8 ? 1.6 ?g/L in these samples. The mean concentrations with standard deviations for each element in biological samples are shown in Table 3. When the arsenic and cadmium levels were compared for each biological samples in cases, a significant negative correlation was found between blood and urine arsenic levels (r= -0.350; p= 0.025). Blood and hair cadmium levels were also significant positive correlated (r= -0.371; p= 0.017).
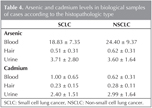
According to the histopathologic type of lung cancer, arsenic and cadmium levels in biological samples except of urine arsenic and blood cadmium were higher in cases with NSCLC than SCLC, however it was not significant statistically (p> 0.05, Table 4).
The mean survival time of cases was 522.3 ? 487.4 (4-1460) days. Mortality rate was 76.4% (n= 55) in all subjects on follow-up. In present study, both of metal levels were not related with mortality, statistically.
DISCUSSION
Eventhough multipl risk factors have been well characterized for lung cancer development chronic exposure to toxic metals may also play role in the pathogenesis. Occupational exposure and expoisoning in the endemic areas to arsenic and cadmium are common because of their wide spread use in industry and their environmental persistence. Although few studies reported that arsenic and cadmium had no relationships to lung cancer, many large epidemiologic studies in pesticide plants, plastics, pigments, batteries, and smelters workers and among tin miner, copper, zinc and lead smelters over the past 50 years have been well defined an increased lung cancer risk in arsenic and cadmium-exposed population, to date (2,16,17). Additionally, chronic poisoning among the general population to these metals by inhalation or oral route is also keep in mind for development of lung cancer beside of occupational exposure (18). Little is known about carcinogenic potential of arsenic and cadmium in general population which usually entail lower levels of exposure. The assessment of past exposure to environmental arsenic and cadmium and their compounds is difficult.
The lungs are target organs of several chemicals including metals by inhalation. Because of these metals have a low solubility in the alveolar regions of the lungs as water, so these poorly soluble compounds can be expected to have a long residence time in the lungs, long half-life values are observed for these metals in liver and kidney (19,20). Although arsenic and cadmium levels may be measured in tumor tissue of cases with lung cancer, the traditional approach is based on the determination of toxic metals and their metabolites in biological samples such as urine, human blood, and hair due to their urinary excretion and bioavailability (8,21,22). The present study provided data on arsenic and cadmium levels in biological samples obtaining from a small study group who diagnosed lung cancer in a single center from Turkish population.
Whereas occupational exposure is well-described risk factor for arsenic and cadmium exposure; there was no found a relation between occupational history and metal levels of cases in this study, this result may be related with any cases had occupational history including arsenic and/or cadmium poisoning (23).
The relationship of smoking and lung cancer have been known very long time (24,25). When the association of toxic metals and smoking is over viewed, tobacco smoke is the main factor affecting the concentration levels of cadmium, lead, and to a lesser extent nickel in the lung tissues of NSCLC patients. Particularly, cadmium elevation in blood, urine, hair and tissue of smokers had been described previously (12,21,26). Cigarette smoke contains measurable amounts of cadmium, and passive smokers living in the same households as smokers have significantly higher blood cadmium levels (27,28). Nonetheless, non-smokers have also been lung cancer due to environmental risk factors and carcinogenic effects of metals, approximately 10-15% of all patients with lung cancer (29). In this study no association was found between arsenic, cadmium levels and smoking history.
According to the histopathology of lung cancer, a few studies evaluating to association of tumor metal levels and lung cancer type have been observed in literature. Guo et al. showed that the carcinogenicity of arsenic on lungs is also cell type-specific. A result of this study including 37.290 lung cancer patients, squamous cell and small cell carcinomas appeared to be related to arsenic ingestion, but not adenocarcinoma (30). Heck et al's study also supported the possibility of an increased risk of small-cell and squamous-cell carcinoma of the lung at lower levels of arsenic exposure (5). In present study, in cases with NSCLC, arsenic and cadmium levels of biological samples were found higher than cases with small cell lung cancer, but not significant statistically. Hair analysis showed high levels for both of arsenic and cadmium although it was insignificant. This may indicate that long term exposure to even small amounts of arsenic and cadmium may affect patients with lung cancer. No relationship was found between the blood cadmium and urine arsenic levels of cases with the type of lung cancer; it can be associated that blood and hair analysis of these metals has detects immediate exposure (31,32). However, we thought that these results might be significant with the larger studies.
Previous analysis of lung cancer mortality in these metals identified excess lung cancer mortality. According to the Third National Health and Nutrition Examination Survey (NHANES III), cadmium appears to be associated with overall cancer mortality in men and women (33). Another study (Park et al.) was also showed that cadmium was an independent factor on mortality of lung cancer (34). Garc?a-Esquinas et al. showed that low to moderate exposure to inorganic arsenic was associated with increased mortality for lung cancer, and the other studies were confirmed that arsenic exposition was also related the cumulative chance of survival declined significantly in patients with lung cancer compared with the controls (35,36). However Sorahan et al. and Li et al. were not found any relationship between cadmium exposure and lung cancer mortality (37-39). We could not show any relation between cadmium and arsenic levels in biological samples and mortality in lung cancer cases.
Epidemiologic studies showed that heavy metals contamination of groundwater, surface water, air and foods is major public health hazard in Turkey as it has been in all of the world (40). Although the association of toxic metals exposure and lung cancer has been well defined in most of countries, there are few reports showing the association of metal levels and lung cancer from Turkey. Cobanoglu et al. were conducted a study in 50 cases (30 lung cancer and 20 healthy human) from Turkish population and they were observed that serum cadmium value was higher in lung cancer but it was not statistically significant (7). A limitation of this study was performed in small number of cases at a single center. Other limitation was that the past environmental exposure dose of arsenic and cadmium for each patient was unknown.
In conclusion, we did not observe any significant relationship between the characteristics of lung cancer and arsenic and cadmium levels in biological samples. Larger studies are necessary for the evaluation of toxic metal levels' affects to lung cancer in general population from Turkey. The recognition of toxic metal exposure in patients diagnosed lung cancer by measurements of metal levels in biological samples is important, thus it can lead to actions to reduce exposure. In order to protect public health for possible polluting toxic elements, it is important to know the concentrations of these elements in the human body.
AUTHORS' CONTRIBUTIONS
?S and ND conceived the study, prepared the data, conducted the analysis, and drafted manuscript. VAT, ZK, and TS analyzed the metal analysis, conducted the analysis, statistics and writing. All authors read and approved the final manuscript.
Acknowledgment
This work was financially supported by T. R. Prime Ministry State Planning Organization and Research Fund of Ankara University (Grant number 2003K1201902).
CONFLICT OF INTEREST
None declared.
REFERENCES
- Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 2008;82(8):493-512.
- ATSDR (Agency for Toxic Substances and Disease Registry) Arsenic Toxicological Profile. Atlanta, GA: ATSDR. 2007. Available at: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=22&tid=3 [accessed 2 October 2012].
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological Profile for Cadmium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012 Sep. Available from: http://www.ncbi.nlm.nih.gov/books/NBK158838/
- Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol 2004;198(3):243-52.
- Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect 2009;117(11):1718-23.
- Waalkes MP. Cadmium carcinogenesis. Mutat Res 2003;533(1-2):107-20.
- Cobanoglu U, Demir H, Sayir F, Duran M, Mergan D. Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pac J Cancer Prev 2010;11(5):1383-8.
- Wadhwa SK, Kazi TG, Kolachi NF, Afridi HI, Khan S, Chandio AA, et al. Case-control study of male cancer patients exposed to arsenic-contaminated drinking water and tobacco smoke with relation to non-exposed cancer patients. Hum Exp Toxicol. 2011;30(12):2013-22. doi: 10.1177/0960327111408154. Epub 2011 May 9.
- Garc?a-Esquinas E, Pollan M, Umans JG, Francesconi KA, Goessler W, Guallar E, et al. Arsenic Exposure and Cancer Mortality in a US-based Prospective Cohort: the Strong Heart Study. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1944-53. doi:10.1158/1055-9965.EPI-13-0234-T. Epub 2013 Oct 17.
- Kazi TG, Jalbani N, Kazi N, Janali MK, Arain MB, Afridi HI, et al. Evaluation of toxic metals in blood and urine samples of chronic renal failure patient, before and after dialysis. Ren Fall 2008;30(7):737-45.
- Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Shah AQ, et al. Evaluation of essential trace and toxic elements in biological samples. Biol Trace Elem Res 2011;143(1):20-40.
- Kazi TG, Memon AR, Afridi HI, Jamali MK, Arain MB, Jalbani N, et al. Determination of cadmium in whole blood and scalp hair samples of Pakistani male lung cancer patients by electrothermal atomic absorption spectrometer. Sci Total Environ 2008;389(2-3):270-6.
- Hou J, Chen G, Wang Z. Simultaneous determination of trace cadmium and arsenic in grains using atomic absorption spectrophotometry. Guang Pu Xue Yu Guang Pu Fen Xi 2001;21(3):387-90.
- Afridi HI, Kazi TG, Jamali MK, Kazi GH, Arain MB, Jalbani N, et al. Evaluation of toxic metals in biological samples (scalp hair, blood and urine) of steel mill workers by electrothermal atomic absorption spectrometry. Toxicol Ind Health 2006;22(9):381-93.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al.; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC lung cancer staging project: proposals for the revision of TNM staging groupings in the forthcoming (seventh) edition of the TNM classification of the malignant tumours. J Thorac Oncol 2007;2(8):706-14.
- Mabuchi K, Lilienfeld AM, Snell LM. Lung cancer among pesticide workers exposed to inorganic arsenicals. Arch Environ Health 1979;34(5):312-20.
- Elinder CG, Kjellstr?m T, Hogstedt C, Andersson K, Sp?ng G. Cancer mortality of cadmium workers. Br J Ind Med 1985;42(10):651-5.
- Tokar EJ, Benbrahim-Tallaa L, Waalkes MP. Metal ions in human cancer development. Met Ions Life Sci 2011;8:375-401.
- Krantz A, Dorevitch S. Metal exposure and common chronic diseases: a guide for the clinician. Dis Mon 2004;50(5):220-62.
- J?rup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellstr?m T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health 1983;9(4):327-31.
- De Palma G, Goldoni M, Catalani S, Carbognani P, Poli D, Mozzoni P, et al. Metallic elements in pulmonary biopsies from lung cancer and control subjects. Acta Biomed 2008;79(Suppl 1):43-51.
- Apostoli P. Elements in environmental and occupational medicine. J Chromatogr B Analyt Technol Biomed Life Sci 2002;778(1-2):63-97.
- 't Mannetje A, Bencko V, Brennan P, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, et al. Occupational exposure to metal compounds and lung cancer. Results from a multi-center case-control study in Central/Eastern Europe and UK. Cancer Causes Control 2011;22(12):1669-80.
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J 1950;2(4682):739-48.
- Nordberg GF. Lung cancer and exposure to environmental cadmium. Lancet Oncol 2006;7(2):99-101.
- Gerhardsson L, Nordberg GF. Lung cancer in smelter workers--interactions of metals as indicated by tissue levels. Scand J Work Environ Health 1993;19(Suppl 1):90-4.
- Wysowski DK, Landrigan PJ, Ferguson SW, Fontaine RE, Tsongas TA, Porter B. Cadmium exposure in a community near a smelter. Am J Epidemiol 1978;107(1):27-35.
- El-Agha O, G?kmen IG. Smoking habits and cadmium intake in Turkey. Biol Trace Elem Res 2002;88(1):31-43.
- Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009;15(18):5626-45.
- Guo HR, Wang NS, Hu H, Monson RR. Cell type specificity of lung cancer associated with arsenic ingestion. Cancer Epidemiol Biomarkers Prev 2004;13(4):638-43.
- Lauwerys R, Roels H, Regniers M, Buchet JP, Bernard A, Goret A. Significance of cadmium concentration in blood and in urine in workers exposed to cadmium. Environ Res 1979;20(2):375-91.
- Brima EI, Haris PI, Jenkins RO, Polya DA, Gault AG, Harrington CF. Understanding arsenic metabolism through a comparative study of arsenic levels in the urine, hair and fingernails of healthy volunteers from three unexposed ethnic groups in the United Kingdom. Toxicol Appl Pharmacol 2006;216(1):122-30. Epub 2006 Jun 8.
- Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup Environ Med 2012;69(2):153-6.
- Park RM, Stayner LT, Petersen MR, Finley-Couch M, Hornung R, Rice C. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup Environ Med 2012;69(5):303-9.
- Nakadaira H, Endoh K, Katagiri M, Yamamoto M. Elevated mortality from lung cancer associated with arsenic exposure for a limited duration. J Occup Environ Med 2002;44(3):291-9.
- Lee-Feldstein A. Cumulative exposure to arsenic and its relationship to respiratory cancer among copper smelter employees. J Occup Med 1986;28(4):296-302.
- Sorahan T, Esmen NA. Lung cancer mortality in UK nickel-cadmium battery workers, 1947-2000. Occup Environ Med 2004;61(2):108-16.
- Sorahan T, Lister A, Gilthorpe MS, Harrington JM. Mortality of copper cadmium alloy workers with special reference to lung cancer and non-malignant diseases of the respiratory system, 1946-92. Occup Environ Med 1995;52(12):804-812.
- Li Q, Nishijo M, Nakagawa H, Morikawa Y, Sakurai M, Nakamura K, et al. Relationship between urinary cadmium and mortality in habitants of a cadmium-polluted area: a 22-year follow-up study in Japan. Chin Med J (Engl) 2011;124(21):3504-9.
- Akbulut NE, Tuncer AM. Accumulation of heavy metals with water quality parameters in K?z?l?rmak River Basin (Delice River) in Turkey. Environ Monit Assess 2011;173(1-4):387-95.
Yaz??ma Adresi (Address for Correspondence)
Dr. Nalan DEM?R
Ankara ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
06100 Cebeci, ANKARA - TURKEY
e-mail: dr.ndemir@gmail.com