RESEARC ARTICLE
Doi: 10.5578/tt.3552
Tuberk Toraks 2014;62(2):116-121

Aktif t?berk?lozlu birey ile temas? olmayan ?ocuklarda
QuantiFERON-TB gold in-tube test ile t?berk?lin cilt testinin kar??la?t?r?lmas?
?zge MET?N T?MUR1, G?n?l TANIR1, Fatma Nur ?Z1, G?ls?m ?clal BAYHAN2, T?rkan AYDIN TEKE3, Nilden TUYGUN2
1 Clinic of Infectious Diseases, Dr. Sami Ulus Maternity and Children Health and Diseases Training and Research Hospital,
Ankara, Turkey
1 Dr. Sami Ulus Kad?n Do?um, ?ocuk Sa?l??? ve Hastal?klar? E?itim ve Ara?t?rma Hastanesi, ?nfeksiyon Hastal?klar? Klini?i,
Ankara, T?rkiye
2 Department of Children's Health and Diseases, Dr. Sami Ulus Maternity and Children Health and Diseases Training and
Research Hospital, Ankara, Turkey
2 Dr. Sami Ulus Kad?n Do?um, ?ocuk Sa?l??? ve Hastal?klar? E?itim ve Ara?t?rma Hastanesi, ?ocuk Sa?l??? ve
Hastal?klar? B?l?m?, Ankara, T?rkiye
3 Clinic of Pediatric Infectious Diseases, Dr. Sami Ulus Maternity and Children Health and Diseases Training and
Research Hospital, Ankara, Turkey
3 Dr. Sami Ulus Kad?n Do?um, ?ocuk Sa?l??? ve Hastal?klar? E?itim ve Ara?t?rma Hastanesi, ?ocuk ?nfeksiyon Hastal?klar?
Klini?i, Ankara, T?rkiye
?ZET
Aktif t?berk?lozlu birey ile temas? olmayan ?ocuklarda quantiferon-Tb gold in-tube test ile t?berk?lin cilt testinin kar??la?t?r?lmas?
Giri?: Bu ?al??mada, Bacille Calmette- Guerin (BCG) a??s? bulunan ?ocuklarda latent t?berk?loz infeksiyonu tan?s?nda QuantiFERON-TB gold in-tube test (QFT-GIT) ile t?berk?lin cilt testini (TCT) kar??la?t?rmay? ama?lad?k.
Materyal ve Metod: ?al??mada 2008-2011 y?llar? aras?nda bilinen t?berk?loz temas ?yk?s? olmayan TCT pozitif 81 ?ocuk prospektif olarak de?erlendirildi. Hastalar ya?lar?na, TCT uygulanma nedenlerine, BCG a?? skar say?lar?na ve TCT endurasyonuna g?re gruplara ayr?ld?. ?n-arka, yan akci?er grafileri ve gerek g?r?l?rse bilgisayarl? tomografi uyguland?.
Bulgular: ?al??mada ortalama ya?? 94.8 ? 51.9 ay olan (6-193 ay aras?nda) 48 erkek (%59.3) ve 33 k?z (%40.7) vard?. Altm?? dokuz (%85.2) ?ocukta bir ve 12 (%14.8) ?ocukta iki BCG skar? mevcuttu. TCT endurasyon ?aplar? 65 (%80.2) ?ocukta? 15-19 mm ve 16 (%19.8) ?ocukta ≥ 20 mm idi. ?al??maya al?nan 12? (%14.8) hastada QFT-GIT pozitfli?i bulundu. QFT-GIT pozitif hastalar ??l? antit?berk?loz tedavi veya izoniazid (INH) ile tedavi edildi. ?? y?ll?k ?al??ma d?neminde, antit?berk?loz ila?larla tedavi edilmeyen ?ocuklar aras?nda t?berk?loz hastal??? saptanmad?.
Sonu?: BCG a??s?n?n rutin olarak uyguland??? ?lkelerde, ?zellikle d???k riskli ?ocuklarda, latent t?berk?loz infeksiyonu yanl?? pozitif tan? ve tedavisini dolay?s?yla ila? yan etkilerini azaltabilece?inden pozitif TCT sonu?lar?n?n interferon-gama (IFN-γ) tabanl? testlerle konfirme edilmesini ?nermekteyiz.
Anahtar kelimeler: Latent t?berk?loz infeksiyonu, t?berk?lin cilt testi, QuantiFERON-TB gold in-tube test, Bacille Calmette-Guerin a??s?, interferon-γ
SUMMARY
Comparison of QuantiFERON-TB gold in-tube test with tuberculin skin test in children who had no contact with active tuberculosis case
Introduction: In this study, we aimed to compare QuantiFERON-TB gold in-tube test (QFT-GIT) and tuberculin skin test (TST) as a diagnosis of latent tuberculosis infection in the children with Bacille Calmette-Guerin (BCG) vaccine.
Materials and Methods: We evaluated 81 children in the study who have positive TST result without a known history of tuberculosis? contact from 2008 to 2011 prospectively. Patients were separated into groups according to their ages, the reason of TST application, number of BCG vaccination scars and diameter of TST induration. Posteroanterior, lateral chest radiographies and computerized tomography, if necessary, were performed.
Results: The study consists of 48 (59.3%) boys and 33 (40.7%) girls with a mean age of 94.8 ? 51.9 months (ranged from 6 to 193 months). Sixty nine (85.2%) children had one and 12 (14.8%) had two BCG vaccination scars. The TST induration diameters were 15-19 mm in 65 (80.2%) children and ≥ 20 mm in 16 (19.8%) children. QFT-GIT positivity was found in 12 (14.8%) of the evaluated patients. QFT-GIT positive patients were treated with triple anti-tuberculosis regime or isoniazid (INH). In three years period of study, there were no tuberculosis disease observed among the children who had not been treated with anti-tuberculosis drugs.
Conclusion: As a result of the study it is suggested to confirm positive TST results with tests based on interferon-gamma (IFN-γ) because it can reduce false positive diagnosis and treatment of latent tuberculosis infection, thus adverse reactions of drugs, in countries where BCG vaccination is routinely recommended especially for low risk children.
Key words: Latent tuberculosis infection, tuberculin skin test, QuantiFERON-TB gold in-tube test, Bacille Calmette-Guerin vaccine, interferon-γ
INTRODUCTION
The World Health Organization (WHO) estimates that 8 to 10 million people develop tuberculosis (TB) in the world every year. In developing countries, approximately 15% of the total TB cases are children younger than 15 years old. Approximately 1.3 million cases of TB disease and 400.000 TB related deaths occur annually among children younger than 15 years old (1). Incidence of TB was 29 per 100.000 population per year, prevalence of TB was 42 per 100.000 population and 17.402 TB case reported in Turkey according to WHO records 2009 (2). Rapid diagnosis and treatment of TB are important for both morbidity/mortality of the patients and disease control in a population. Strict contact tracing and use of preventive chemotherapy is important to reduce TB-related suffering of children. Untreated latent TB infection (LTBI) in children provides the seed of the epidemic for the next generation. It is estimated that one third of the world's population has LTBI. This global prevalence of LTBI was estimated predominantly on the basis of data obtained from the tuberculin skin test (TST) survey (3). Although TST has some limitations, it remains the most acceptable method for diagnosing LTBI and has been used for years. This test is not gold standard method because it gives false positive and negative results in many circumstances. In recent years, identification of Mycobacterium tuberculosis-specific antigens which are encoded within the region of difference 1, has led to the development of new commercial immunodiagnostic tests that are able to distinguish the production of interferon-gamma (IFN-γ) in response to M. tuberculosis infection induced by either Bacille Calmette-Guerin (BCG) vaccine or environmental mycobacteria (4). QuantiFERON-TB gold in-tube (QFT-GIT) Test detects the in vitro cell-mediated immune response to M. tuberculosis with the method of enzyme-linked immunosorbent assay (ELISA) by using early secretor antigenic target 6 (ESAT-6), culture filtrate protein 10 (CFT-10) and TB 7.7 (5). QFT-GIT test is only interferon- release assay (IGRA) test which has been approved by American Food and Drug Administration in 2005 (6). Although IGRAs have been evaluated among adults, studies on children are limited yet. We conducted a study in 81 BCG vaccinated/TST positive children who were evaluated for LTBI by using QFT-GIT.
Materials and Methods
The study population consisted of 81 children who were evaluated for LTBI between 2008 and 2011. Demographic and clinical data, BCG vaccination status (the presence of typical BCG scar), TST result, and reasons for testing were noted by using a standard form. Inclusion criteria for the study were as follows:
1. Children with positive TST result
2. Children without a history of contact with a TB case
3. Active TB case in the household was not detected through the family screening
4. Children having no medical reason for immunosuppression.
The study protocol was approved by the local hospital ethics committee.
Reasons of TST application were school or nursery school screening, chronic cough and recurrent pneumonia. There was also one child who had been diagnosed acute appendicitis and his postoperative material revealed, caseification granuloma. Because of the QFT-GIT test had not been used in routine practice in the study period, there was not any case who was diagnosed TB disease by using this method solely. The children who had diagnosed TB disease were not excluded from the study because they had no contact with active TB case. School or nursery school screening is defined as testing children for only administrative reasons. Chronic cough is defined as a daily cough lasting for ≥ 4 weeks (7). Recurrent pneumonia is defined as two or more episodes in a single year or three or more episodes ever (8).
All children underwent a TST with 5 TU of purified protein derivative, according to intradermal Mantoux method. When interpreting a TST result, the widest diameter of induration, not erythema, was measured in millimetres after 72 hours by trained physician or nurses. All children had at least one BCG scar. TST was considered as positive if an induration was ≥ 15 mm in diameter, regardless of BCG vaccination scar numbers.
Peripheral blood samples for QFT-GIT tests were taken in the laboratory, where they were processed by trained physicians and performed according to manufacturer's instructions. For each children, total 3 mL whole blood was taken, then blood was collected in three special tubes: gray- (negative control, ??nil''), red- (test tube), and purple-cap (positive control; mitogen-coated) tubes. Test tube is specially designed for blood collection which is coated with M. tuberculosis-specific antigens (ESAT-6, CFP-10, and a portion of TB 7.7). Once blood was collected it is essential to provide adequate shaking for antigens to dissolve. They were incubated at 37˚C for 16 to 24 hours and centrifugation at 3000 g for 15 minutes, then plasma was separated. The amount of IFN-γ was measured by using the QFT ELISA. A positive result was defined if the difference in the IFN-γ levels between the test tube and negative control is greater than or equal to 0.35 IU/mL and is greater than 25% of the nil value. Also for determinate results, nil control must be less than 8.0 IU/mL.
Posteroanterior, lateral chest radiographies and computerized tomography (CT), if necessary, were performed to patients. All chest radiographies were read by a radiologist and the findings were considered normal or abnormal (presence of hilar, mediastinal lymphadenopathy, parenchymal infiltrate, pleural-pericardial effusion).
Latent tuberculosis infection was defined both TST and QFT-GIT test positive in a child who had no abnormality on chest X-ray. Active TB disease was defined both TST and QFT-GIT test positive in a child who had symptoms of TB disease and/or abnormal findings on chest radiograph, CT or proven M. tuberculosis culture, PCR or histopathological examination. The children whose QFT-GIT test were negative were evaluated as having no LTBI or TB disease if they had no risk factors and symptoms of TB disease or if they had negative results after all investigations and they had confirmed alternative diagnosis and complete recovery without specific anti-TB treatment. These children were followed up for three years as outpatients with three months intervals. At the follow up visits, we questioned about symptoms such as cough, fever, and weight loss.
Statistical analyses
Statistical analyses were undertaken with SPSS version 15.0. QFT-GIT test recorded as positive or negative based on IFN-γ concentration cut-off value of 0.35 IU/mL. The overall results of this study were expressed as percentages for categorical variables, means ? SD, and as medians for continuous variables. Correlations between categorical variables were assessed by using chi-square tests. Differences between groups were evaluated by using Mann Whitney test. Odds ratio was given for examining the relationship between TST and QFT-GIT positivity. A p value of ≤ 0.05 was considered as significant.
Results
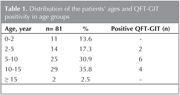
A total of 81 BCG-vaccinated children were enrolled in study. They underwent TST and QFT-GIT assay. The population of the study consisted of 48 (59.3%) boys and 33 (40.7%) girls with a mean age of 94.8 ? 51.9 months (ranged from 6 months to 193 months). Distributions of the patients' ages and QFT-GIT positivity in age groups are demonstrated in Table 1. Sixty nine of (85.2%) 81 participants had one BCG scar, 12/81 (14.8%) had two BCG scars. Sixty five (80.2%) children had TST indurations of 15-19 mm, 16 (19.8%) children had ≥ 20 mm. The reasons of TST application were chronic cough in 54/81 (66.7%), recurrent pneumonia in 10/81 (12.3%), school-nursery school screening in 9/81 (11.1%), and other reasons in 8/81 (9.9%) of the children. The other reasons were caseification in postoperative material (one patient), pleural/pericardial effusion (one patient), fever of unknown origin (one patient), cervical lymphadenopathy (three patients), dermatitis (one patient) and chronic abdominal pain (one patient).
There were no statistically significant relationship with age and QFT-GIT positivity (p= 0.298). Sex distribution did not show any relation to QFT-GIT positivity also (p= 0.944). Among the 65 patients with a TST induration of 15-19 mm, 8 (12.3%) of them and 16 patients with TST induration of ≥ 20 mm, 4 (25%) of them had positive QFT-GIT result. Increasing TST induration increases the possibility of QFT-GIT positivity 2.37 times (95% CL 0.61-9.18).
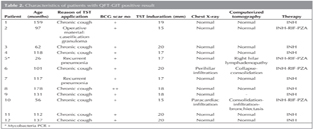
Chest X-ray investigations were applied to all of the study population, a chest CT scan was performed if necessary. Eight of the 12 QFT-GIT positive children had normal chest radiographies and CT findings. These eight children were judged as LTBI and were treated with isoniasid (INH) for six months. One QFT-GIT positive patient was diagnosed as having abdominal TB according to histopathological findings in his postoperative material. Two of the 12 QFT-GIT positive children had chronic cough and another one had recurrent pneumonia. These three children were diagnosed as pulmonary TB because of the compatible findings on chest radiograph and CT scan. The four patients, who had TB disease, were treated with INH, rifampicin (RIF) and pyrazinamide (PZA). Characteristics of QFT-GIT positive patients are summarized in Table 2.
In three years period of study, there were no TB disease observed among the children who had not been diagnosed as having LTBI and had not been treated with anti-TB drugs.
Discussion
Tuberculosis disease is still an important cause of morbidity in children in our country and in the world. Detection and treatment of LTBI are key strategies for controlling TB spread in the community. The accurate diagnosis of LTBI reduces the risk of progression to severe disseminated disease especially in children.
The risk of progression from LTBI to active TB is inversely related to age; studies had shown that in 40% to 50% of infants with untreated TB infection disease develops within 1 to 2 years (9). Because of the younger children fail to contain the spread of intracellular pathogens as a consequence of an impaired T-cell response. It was considered that QFT tests were negatively affected by young age particularly < 4 years of age old in terms of phytohaemagglutinin-produced IFN-γ production (10). A recent study of 227 children between 0 to 15 years of age also reported a statistically significant correlation between mitogen response in the QFT-GIT assay and age (11). In contrast to previous study, in a recent study QFT-GIT had been performed for 50 children with TB, prior to administrating anti-TB treatment and authors evaluated age-related effects of test. They reported that significantly higher IFN-γ responses to TB-specific antigens were associated with younger age, but no difference was found with mitogen responses. It was concluded that QFT-IT responses to TB-specific antigens are not related with young age (12). In our study, although the relationship between positive QFT-GIT results and age are not statistically significant, QFT-GIT results are negative especially for < 5 years old children. This may be due to the fact that our study's relatively small size.
T-cell IGRAs have been shown to be effective tools for the detection of M. tuberculosis infection, offering an enhanced specificity compared to TST (13). It has been found that the TST + /IGRA- results were associated with prior BCG vaccination (14). In a recent study, 2063 (51.6%) BCG-vaccinated and 1933 (48.4%) BCG-non-vaccinated total 3996 children were screened for LTBI. Authors found that TST positivity was more likely in vaccinated children than non-vaccinated children (5.7% vs. 0.2%, p< 0.001). Among 65 BCG-vaccinated TST-positive children who underwent a QFT-GIT, only 5 (7.7%; 95% CI: 2.5%, 17.0%) were QFT-GIT positive (15). The agreement between the QFT-GIT and the TST in BCG-vaccinated and non-vaccinated children with LTBI or TB disease, with and without risk factors for TB was compared in a multicentre, prospective study in immunocompetent children. It was found that agreement between tests in BCG-vaccinated children was lower than in non-vaccinated cases. Tests in children exposed to TB showed better agreement than in children not exposed to TB (16). Because BCG is routinely implemented to all two months old infants in Turkey, we did not have a comparison group of BCG-non-vaccinated children. Since we had not have BCG-non-vaccinated or TB exposed comparison groups, sensitivity and specificity of QFT-GIT over TST have not been established. It was found that only 15% of TST positive BCG vaccinated children were QFT-GIT positive in our study.
It was found that a TST of ≥ 15 mm was more likely to be associated with a positive QFT-GIT than a TST of 10 to 14 mm (15). Using QuantiFERON-TB Test as the reference test, a TST cut-off point of ≥ 5 mm had been reported having 100% sensitivity and 93% specificity in children without BCG. In contrast in BCG-vaccinated children, the TST cut-off point of ≥ 10 mm and ≥ 15 mm had been found having poorer specificity (86%) and reduced sensitivity (60%) respectively (17). And also we found that increasing TST induration increases the possibility of QFT-GIT positivity but there was no statistically significant result.
Routine TST screening of children before entering primary school or nursery school is often practiced in some countries and many of these children were BCG-vaccinated in infancy period. The reliability of the TST in such children is unknown. False positive results because of BCG vaccination causes further investigations for active TB disease. This may result in the treatment of some children who actually have not been infected. In this study, all of nine children who were applied TST because of school administration had no positive QFT-GIT result. TST screening should not be used for screening children with a low probability of infection, because false positive results may cause unnecessary medical evaluation and treatment.
There is insufficient evidence to recommend the use of serological tests for active TB diagnosis. The accuracy of two IGRAs was evaluated in 395 suspected TB patients (27% HIV-infected) in South Africa. Authors thought that in a high-burden setting, IGRAs do not have value as rule-in or rule-out tests for active TB when used alone (18). It had been recommended that although IGRAs can be used as evidence of LTBI infection in children, appropriate specimen collection and microbiological confirmation of TB disease should remain a priority (19). In the present study QFT-GIT results strengthened the diagnosis of TB disease in four patients despite the lack of the precise history of any contact to TB.
In conclusion, acknowledging the limitations, the results of this study suggests that confirmation of positive TST results with QFT- GIT test may enhance the accuracy of diagnosing both active TB and LTBI, particularly among BCG vaccinated children. The correct diagnosis of LTBI prevents unnecessary treatment and treatment complications.
CONFLICT of INTEREST
None declared.
REFERENCES
- Lighter J, Rigaud M. Diagnosing childhood tuberculosis: traditional and innovative modalities. Curr Probl Pediatr Adolesc Health Care 2009;39:61-88.
- World Health Organization (WHO). Tuberculosis Country Profil. Turkey. Epidemiology and strategy. Generated: October 20, 2011. Available from: http://www.who.int/tb/country/data/profiles/en/index.html
- Legesse M, Ameni G, Mamo G, Medhin G, Bjune G, Abebe F. Community-based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test. BMC Infect Dis 2011;11:89.
- Hoff ST, Abebe M, Ravn P, Range N, Malenganisho W, Rodriques DS, et al. Evaluation of Mycobacterium tuberculosis-specific antibody responses in populations with different levels of exposure from Tanzania, Ethiopia, Brazil, and Denmark. Clin Infect Dis 2007;45:575-82.
- Kariminia A, Sharifnia Z, Aghakhani A, Banifazl M, Eslamifar A, Hazrati M, et al. Comparison of QuantiFERON TB-G-test to TST for detecting latent tuberculosis infection in a high-incidence area containing BCG-vaccinated population. J Eval Clin Pract 2009;15:148-51.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobac-terium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54(RR-17):1-141.
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest 2006;129(Suppl 1):S260-S83.
- Sectish TC, Prober CG. Pneumonia: pathogenesis. In: Behrman RE, Kliegman RM, Jenson HB (eds). Nelson Textbook of Pediatrics. 18th ed. Philadelphia 2008:1795-800.
- Khan EA, Starke JR. Diagnosis of tuberculosis in children: increased need for better methods. Emerg Infect Dis 1995;1:115-23.
- Bergamini BM, Losi M, Vaienti F, D'Amico R, Meccugni B, Meacci M, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 2009;123:419-24.
- Chun JK, Kim CK, Kim HS, Jung GY, Lee TJ, Kim KH, et al. The role of a whole blood interferon-gamma assay for the detection of latent tuberculosis infection in Bacille Calmette-Gu?rin vaccinated children. Diagn Microbiol Infect Dis 2008;62:389-94.
- Markova R, Drenska R, Minchev P, Todorova Y, Ciccozzi M, Amicosante M. Association of age with the level of response in the QuantiFERON-TB Gold In-Tube assay for children with active tuberculosis. New Microbiol 2011;34:81-5.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177-84.
- Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 2001;286:1740-7.
- Jacobs S, Warman A, Richardson R, Yacoub W, Lau A, Whittaker D, et al. The tuberculin skin test is unreliable in school children BCG-vaccinated in Infancy and at low risk of tuberculosis infection. Pediatr Infect Dis J 2011;30:754-8.
- M?ndez-Echevarr?a A, Gonz?lez-Mu?oz M, Mellado MJ, Baquero-Artigao F, Bl?zquez D, Pen?n M, et al.; Spanish Collaborative Group for the Study of the QuantiFERON-TB GOLD Test in Children. Interferon-{gamma} release assay for the diagnosis of tuberculosis in children. Arch Dis Child 2011.
- M?ndez-Echevarr?a A, Gonz?lez-Mu?oz M, Mellado MJ, Baquero-Artigao F, Vecino R, P?rez E; Spanish Collaborative Group for the Study of QuantiFERON-TB GOLD Test in Children. Optimizing interpretation of the tuberculin test using an interferon-gamma release assay as a reference standard. Pediatr Infect Dis J 2011;30:426-8.
- Ling DI, Pai M, Davids V, Brunet L, Lenders L, Meldau R, et al. Are interferon-{gamma} release assays useful for diagnosing active tuberculosis in a high-burden setting? Eur Respir J 2011;38:649-56.
- Ling DI, Zwerling AA, Steingart KR, Pai M. Immune-based diagnostics for TB in children: what is the evidence? Paediatr Respir Rev 2011;12:9-15.
Yaz??ma Adresi (Address for Correspondence)
Dr. ?zge Met?n T?mur
Dr. Sami Ulus Kad?n Do?um,
?ocuk Sa?l??? ve Hastal?klar? E?itim ve
Ara?t?rma Hastanesi,
?nfeksiyon Hastal?klar? Klini?i,
ANKARA - TURKEY
e-mail: drozgemetintimur@gmail.com