RESEARC ARTICLE
Doi: 10.5578/tt.6493
Tuberk Toraks 2014;62(1):12-21

Acil Servislerde Pulmoner Tromboemboli ?n Tan?s? ile Gereksiz Tetkik Yap?lmas?
Wells Skoru ve Pulmoner Emboli Ekartasyon Kriterleri ile ?nlenebilir mi?
M?ge AYDO?DU1, Nazl? TOPBA?I S?NANO?LU2, Nurettin ?zg?r DO?AN3, ?pek K?v?lc?m O?UZ?LGEN1,
Ahmet DEM?RCAN4, Fikret B?LD?K4, Numan Nadir EK?M1
1 Gazi ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Ankara, T?rkiye
1 Department of Chest Diseases, Faculty of Medicine, Gazi University, Ankara, Turkey
2 ?orum Osmanc?k Devlet Hastanesi, G???s Hastal?klar? Klini?i, ?orum, T?rkiye
2 Clinic of Chest Diseases, Corum Osmancik State Hospital, Corum, Turkey
3 Ankara Etlik ?htisas E?itim ve Ara?t?rma Hastanesi, Acil Anabilim Dal?, Ankara, T?rkiye
3 Department of Emergency, Ankara Etlik Speciality Training and Research Hospital, Ankara, Turkey
4 Gazi ?niversitesi T?p Fak?ltesi, Acil Anabilim Dal?, Ankara, T?rkiye
4 Department of Emergency, Faculty of Medicine, Gazi University, Ankara, Turkey
?ZET
Acil Servislerde Pulmoner Tromboemboli ?n Tan?s? ile Gereksiz Tetkik Yap?lmas? Wells Skoru ve Pulmoner Emboli Ekartasyon Kriterleri ile ?nlenebilir mi?
Giri?: Pulmoner emboli (PE) tan?s?n? atlaman?n do?urabilece?i olumsuz sonu?lar nedeniyle acil servise nefes darl??? veya pl?retik g???s a?r?s?yla ba?vuran pek ?ok hastaya PE yanl?? ?n tan?s? ile gereksiz tan?sal testler uygulanmaktad?r. Bu ?al??man?n amac?, PE ?n tan?s? ile yap?lan a??r? tetkik oran?n? ve nedenlerini belirlemek; Wells ve Pulmoner Emboli Ekartasyon Kriterleri (PERC) kullan?larak bu oran?n azalt?l?p azalt?lamayaca??n? ara?t?rmakt?r.
Hastalar ve Metod: ???nc? basamak bir ?niversite hastanesinin acil servisinde ger?ekle?tirilen bu retrospektif g?zlemsel kohort ?al??mada PE ??phesiyle tetkik planlanan t?m hastalar ?al??maya dahil edildiler. Tetkik sonu?lar?na g?re PE (+) ve PE (-) olarak iki gruba ayr?larak demografik ve laboratuvar ?zellikleri, tetkik sonu?lar?, Wellls ve PERC skorlar? a??s?ndan kar??la?t?r?ld?lar.
Bulgular: ?al??maya dahil edilen toplam 108 hastan?n 53 (%49)'?ne PE (+) tan?s? koyuldu ve gereksiz tetkikin 55 (%51) hastada yap?ld??? belirlendi; PE (-). Y?ksek Wells skorunun (> 6) sensitivitesi %43, spesifisitesi %78, pozitif prediktif de?eri %66, negatif prediktif de?eri %59 olarak bulundu. PERC de?erlendirmesinin sadece be? hastada negatif oldu?u (toplam sekiz kriterin de kar??land???) bulundu. Testin sensitivitesi %98, spesifisitesi %7, pozitif prediktif de?eri %50, negatif prediktif de?eri %80 olarak de?erlendirildi. PERC kriterlerini olu?turan alt ba?l?klar ayr? ayr? PE'yi ekarte etme g??leri a??s?ndan de?erlendirildi?inde ?bacakta ?i?me, ?ap fark? olmamas?? ve ?daha ?nce ge?irilmi? derin ven trombozu veya PE ?yk?s? olmamas?? kriterleri ile PE tan?s? aras?nda istatistiksel anlaml? negatif korelasyon belirlendi (s?ras?yla; p= 0.001, r= -0.325 and p= 0.013, r= -0.214).
Sonu?: Acil servislerde PE ?n tan?s?yla a??r? tetkik yap?lmas? g?n?m?zde halen ?nemli bir sorundur. Bunu ?nlemek i?in g?nl?k pratikte kullan?lan Wells skoru, PERC skoru gibi klinik tahmin skorlar? yetersiz kalabilmektedir. Bu skorlar geli?tirilmeli veya kombine edilerek kullan?lmalar? y?n?nde daha ileri ?al??malar planlanmal?d?r.
Anahtar kelimeler: A??r? tetkik, pulmoner emboli, pulmoner emboli ekartasyon kriterleri, PERC, Wells skoru
SUMMARY
Wells Score and Pulmonary Embolism Rule Out Criteria in Preventing Over Investigation of Pulmonary Embolism in Emergency Departments
Introduction: Unnecessary diagnostic tests are usually ordered to most of the patients with dyspnea or pleuritic chest pain, because of the worse outcomes of missed diagnosis of pulmonary embolism (PE). To identify rates and causes of over investigation for PE and to search whether it was possible to reduce this over investigation by using Wells score and Pulmonary Embolism Rule Out Criteria (PERC).
Materials and Methods: A retrospective observational cohort study performed in an emergency department of a tertiary care university hospital. All patients who were ordered diagnostic with the suspicion of PE were included in the study. They were grouped into two as PE (+) and PE (-) and compared.
Results: Among 108 patients, 53 (49%) were diagnosed as PE (+) and overdiagnosis was present in 55 (51%) patients i.e., PE (-). The sensitivity of high Wells score was 43%, specificity 78%, positive predictive value 66% and negative predictive value 59%. PERC criteria found to be negative (when all of the eight criteria were fulfilled) in only five patients. The sensitivity of the test was 98%, specificity 7%, positive predictive value 50%, negative predictive value 80%. When individual parameters of PERC were evaluated solely for the exclusion of PE; "no leg swelling" and "no previous deep venous thrombosis or PE history" were found significantly negatively correlated with PE diagnosis (p= 0.001, r= -0.325 and p= 0.013, r= -0.214 respectively).
Conclusion: Over investigation of PE in emergency departments still remains as an important problem. In order to prevent this, the clinical prediction rules must be developed further and their use in combination should be searched in future studies.
Key words: Over investigation, pulmonary embolism, pulmonary embolism rule out criteria, PERC rule, Wells score
INTRODUCTION
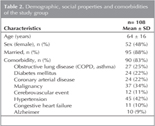
Pulmonary embolism (PE) is a common, frequently undiagnosed and potentially fatal cardiovascular emergency (1). In the United States annually over 110 million patients seek emergency department care and approximately 10 million of these patients have complaints of dyspnea, chest pain or both (2). An estimated 600.000 cases of PE are diagnosed in United States each year (3). But there are also many undiagnosed, missed PE cases because of the nonspecific nature of PE symptoms such as dyspnea, chest pain, syncope and fever. Especially in cases with fever PE can be frequently misdiagnosed as pneumonia (4). Other possible causes for misdiagnosis of PE are chronic obstructive pulmonary disease (COPD) exacerbation, congestive heart failure, acute coronary syndrome, aortic dissection, cerebrovascular event and pleuritis (5). Autopsy studies have revealed that PE follows acute coronary syndrome as the second most common cause of unexpected death in outpatients (6,7).
PE diagnosis is frequently made by being suspicious about the disease. Depending on the clinical and laboratory properties and the medical histories of the patients, most of the PE cases are diagnosed in the emergency departments either by a pulmonologist or an emergency physician (8). Because of the worse outcomes of missed diagnosis of PE such as death or chronic thromboembolic pulmonary hypertension, emergency department physicians and also pulmonologists usually order unnecessary diagnostic tests such as D-dimer and computed tomography (CT) angiography in most of the patients with dyspnea or pleuritic chest pain. Besides, several reports have indicated that physicians order a diagnostic test for PE because of the fear of medical malpractice (9,10). In addition to these factors, the wide availability and acceptance of D-dimer and CT angiography may contribute to the high frequency of ordering unnecessary tests for PE in emergency departments (11). D-dimer test, although still being commonly used, has high false positivity rate (12). The consequences of performing PE diagnostic tests by just depending on high D-dimer levels include: increased cost, increased use of emergency department and hospital resources, increased risk of radio contrast agent associated acute renal failure and increased risk of future malignancies depending on the exposure to CT associated radiation (13). Hence, in emergency departments both the over investigation and misdiagnosis of PE should be prevented. For this purpose some scores have been developed such as Wells score and Pulmonary Embolism Rule Out Criteria (PERC) by combining some clinical properties and possible risk factors (14,15). Wells score is being commonly used in our emergency department, but we have not yet implemented the PERC to our daily practice. In this retrospective study, we aimed to identify rates and causes of over investigation for PE in our emergency department. As a secondary outcome we also aimed to determine whether it was possible to reduce this over investigation rate with the use of Wells score and PERC in daily practice.
MATERIALS and METHODS
This study was designed as a retrospective observational cohort study. It was performed in an emergency department of a tertiary care university hospital i.e. Gazi University Medical Faculty Hospital in Ankara in Turkey, between the years 2009 and 2010. It is a reference hospital in the region that patients are accepted from many other hospitals both from the Ankara district and from many other cities near Ankara. The local ethics committee of our institution had approved the study.
Patient Selection and Evaluation
Inclusion criteria: All patients who were ordered diagnostic tests by an emergency or pulmonary physician in the emergency department with the suspicion of PE were included in the study if they were > 18 years old.
Exclusion criteria: Patients with missing medical records were excluded from the study.
According to our emergency department's daily working routine, all patients were first evaluated by paramedics before being admitted to the emergency department. After being admitted, the patient was evaluated by a resident of emergency department and by an emergency department specialist. If they clinically suspect from PE, further tests were planned for the diagnosis of PE directly by the emergency department physicians. If they had any doubt about the diagnosis they asked for consultation from the pulmonologist. For the clinical evaluation Wells clinical prediction score was used in emergency department by both emergency and pulmonary physicians (14). The ones having moderate or high clinical risk of Wells score were further evaluated with CT angiography. Parameters used for the decision to perform tests for the diagnosis of PE were noted to medical records.
The test that was preferred at first glance for the diagnosis of pulmonary embolism was a 64-slice multislice CT angiography (General Electric?, Light speed VCT, 64 slice) in our university hospital. If CT angiography could not be performed because of morbid obesity or increased renal function tests then venous Doppler ultrasonography (USG) of lower extremities, ventilation-perfusion scintigraphies were used for diagnosis.
Data Collection
Following data were recorded; demographic properties (age, sex, weight), underlying diseases, physical examination, vital findings and symptoms at admission, venous thrombosis risk factors (malignancy, thrombophilia, previous deep venous thrombosis (DVT) or PTE, immobilization, surgical operation or trauma within the last four weeks, pregnancy etc.), D-dimer levels, arterial blood gas analysis values, posteroanterior chest X-ray, electrocardiography, echocardiography (if performed in the emergency department), laboratory tests such as hemostasis tests, complete blood cell count and liver and kidney function tests. Wells scoring, PERC were calculated retrospectively once again (14,15). If possible, causes of ordering diagnostic tests were extracted and recorded form the medical records of the patients. The results of the diagnostic tests were obtained. According to these results patients were grouped into two as PE (+) and PE (-) patient groups and they were compared. Rate of over investigation and factors leading to this over investigation were investigated. The possible effects of clinical prediction scores (Wells and PERC) were assessed for the prevention of over investigation.
Definitions
Venous thromboembolism was diagnosed if patients have (16,17,18);
Pulmonary thrombus identified in multislice CT pulmonary angiography or,
Clinical suspicion of PTE and high probability V/Q lung scan or,
Moderate probability V/Q lung scan and DVT diagnosed in lower extremity venous Doppler USG or,
DVT diagnosis with bilateral lower extremity Doppler USG.
Wells Clinical Prediction Score; was defined in detail in Table 1 (14).
Pulmonary Embolism Rule Out Criteria (PERC); A negative PERC criteria [PERC (-)] requires the clinician to answer no to all of the following eight questions (15):
Is the patient older than 49 years of age?
Is the pulse rate above 99 beats/minute?
Is the pulse oximetry reading < 95% while the patient breathes room air?
Is there a present history of hemoptysis?
Is the patient taking exogenous estrogen?
Does the patient have a prior diagnosis of venous thromboembolism (VTE)?
Has the patient had recent surgery or trauma? (Requiring endotracheal intubation or hospitalization in the previous four weeks.)
Does the patient have unilateral leg swelling? (Visual observation of asymmetry of the calves).
In this study the answers of these questions were extracted from the medical records of the patients and PERC score calculated retrospectively.
Statistics
SPSS for Windows 15.0 software was used for the statistical analysis of the results (SPSS for Windows; Chicago, IL, USA). Results are presented as mean ? SD and percentiles or median (range) values. The independent samples t-test, the chi-square and Mann-Whitney U tests were used for comparison of the categorical and continuous variables. Correlation was investigated with Pearson correlation tests for parametric values. A difference was considered statistically significant when p< 0.05.
RESULTS
Patient Characteristics
A total of 125 patients who were suspected and investigated for PE were evaluated for the study. Sixteen patients were excluded due to missing medical records and one patient due to being pregnant for three months. Thus, study was performed with 108 patients. Demographic characteristics and comorbidities of the whole group were summarized in Table 2.
Diagnostic Tests
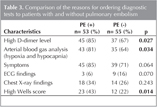
Diagnostic tests were ordered to the entire study group with the suspicion of PE. Thoracic multislice CT angiography was performed in 104 (96%) patients, lower extremity Doppler USG was performed in 4 (4%) patients. All of the patients who had Doppler USG were diagnosed as deep venous thrombosis. Among the whole study group, 53 (49%) were diagnosed as pulmonary embolism [PE (+)], and in 55 (51%) PE was ruled out [PE (-)]. Among the 55 patients without PE, diagnostic tests were ordered by emergency medicine resident in 19 (35%), by pulmonary medicine resident in 30 (55%), by both pulmonary and emergency medicine residents in 6 (11%). Among the 53 patients with PE, diagnostic tests were ordered by emergency medicine resident in 22 (42%), by pulmonary diseases resident in 27 (51%), by both pulmonary medicine and emergency medicine resident in 2 (4%) and by other physicians in 2 (%4) patients. The reasons for ordering diagnostic tests were summarized in Table 3. High D-dimer levels, high Wells score (> 6) and the presence of hypoxia and hypocapnia in arterial blood gas analysis were identified as the significant parameters (p< 0.05). False positivity rates for these parameters were 67% for high D-dimer, 64% for hypoxia and hypocapnia and 22% for high Wells score.
The demographic characteristics, comorbidities, admission symptoms, chest X-rays and electrocardiographies of patients with and without PE were compared in Table 4. Except the cerebrovascular event that was identified more frequent in PE (+) patients, no significant difference was identified in any of these parameters between the two groups (Table 4).
Clinical Prediction Rules and D-dimer
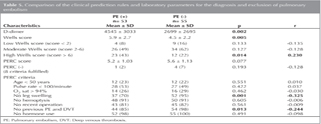
The clinical prediction rules (Wells score and PERC) and D-dimer levels of the two groups were compared in Table 5. In PE (+) patients D-dimer levels and Wells scores were found significantly higher (p< 0.005). High Wells score (score > 6) was found significantly correlated with the PE diagnosis (p= 0.014, r= 0.230). The sensitivity of high Wells score was 43%, specificity 78%, positive predictive value 66% and negative predictive value 59%. PERC found to be negative (when all of the eight criteria were fulfilled) in only five patients. Among those 5 patients, 1 was diagnosed as PE (+), the remaining four were PE (-) (p= 0.193). The sensitivity of the test was 98%, specificity 7%, positive predictive value 50%, negative predictive value 80%, false positivity rate 93% and false negativity rate 2%. When individual parameters of PERC were evaluated solely for the exclusion of PE; "no leg swelling" and "no previous DVT or PE history" were found significantly negatively correlated with the diagnosis of PE (p= 0.001, r= -0.325 and p= 0.013, r= -0.214 respectively) (Table 5).
DISCUSSION
In the last few years, the importance of PE has been understood well, especially by the emergency physicians. With the fear of medical malpractice unnecessary diagnostic tests are being ordered for PE (9,10). Besides, the wide availability and acceptance of D-dimer tests and CT angiography might also contributed to increased rate of ordering excessive diagnostic tests for patients admitted to emergency departments (11,19). For our study population, excessive CT angiography was ordered for 53 (51%) patients. This was lower in the study of Jimenez Castro et al. as 29%; but much higher, varying between 80-93%, in many studies searching about the utility of PERC in low risk patient populations (20,21,22,23).
These excessive diagnostic tests not only increase the cost and length of stay in crowded emergency departments, but also have probable worse effects on patients such as radio contrast related renal failure or increased risk of malignancy due to radiation exposure (24). Hence, we searched the reasons of ordering excessive diagnostic tests from the medical records and compared the characteristics of the patients with and without PE. The high D-dimer level, high Wells score and hypoxia and hypocapnia in arterial blood gas analysis were found as significant parameters for ordering excessive CT angiography. Low Wells score excluded PE cases successfully, however in four patients with low Wells score, acute PE was identified in CT angiography. On the other hand, ECG and chest X-ray findings were remained less important in decision making. Among the ECG findings only the ischemia was identified higher in PE (+) patients and this may be explained by severe hypoxemia leading to the development of ischemic findings. No any demographic parameters was identified for differentiating PE (+) and (-) patients. Except the cerebrovascular event that was identified higher in PE (+) patients, other underlying comorbidities also had no effect on over investigation of PE.
In order to prevent this excessive diagnostic testing, many clinical prediction rules have been developed. The Wells score is the most widely used prediction rule for PE, derived first in year 1998 and then validated in a group of 247 patients where the rates of PE for low, intermediate and high clinical probability were 2%, 18.8% and 50% respectively (14). The association of a low clinical probability of PE and a low D-dimer concentration has been shown to safely rule out PE (25). In 2004, Kline JA, et al. derived the PERC, an eight factor decision rule to support the decision not to order a diagnostic test for PE in patients for whom the clinician already had a low clinical suspicion for PE (15). From the year 2004, many studies have been performed to validate the utility of PERC (21,22,23,26,27,28,29). They all commented that the PERC rule is best suited to a patient population with a prevalence of < 10% PE. In this low prevalence population they assessed the sensitivity of the rule as 100%, specificity varying between 16%-33%, negative predictive value as 100% (21,22,23,26,27,28,29). In addition to the necessity of low prevalence population, PERC should only be applied to low risk patients, so an application of another clinical decision rule such as Wells criteria is necessary to evaluate the clinical risk. Only Wolf, et al. evaluated this rule not only in low risk population but for all risk levels of PE and stated that PERC was highly sensitive (100%) with an excellent negative predictive value (100%) for a population of all risk groups (22). They identified the obvious disadvantage of PERC as it would have missed 3 cases of PE (22). In comparison, using the Wells clinical probability score in combination with D-dimer missed only two cases of PE (26,30). As Singh B, et al., explained in their review that the existing literature suggest consistently high sensitivity and low but acceptable specificity of the PERC to rule out PE in patients with low pretest probability (31). When the pretest probability is low PERC are highly sensitive in predicting PE and D-dimer testing is thus unnecessary. In their review the two of the included studies [Hugli, et al. (32) and Righini, et al. (33)] reported a higher frequency of missed PE and have raised a concern about the reliability of PERC. But their higher failure rate was thought to be likely resulting from the higher PE prevelance observed in their European settings (32,33). Hugli, et al. reported that PERC rule alone or even when combined with the revised Geneva score cannot safely identify very low risk patients in whom PE can be ruled out without additional testing, at least in populations with a relatively high prevelance of PE (32). It should always be remembered that the PERC rule was developed for use in low probability settings. Similar to Hugli et al. we also identified high PE prevelance in our study as 49%.
We retrospectively analyzed our results for PERC and found it to be negative (when all of the 8 criteria were fulfilled) in only 5 (5%) patients. We thought that this low number of patients with PERC (-) can be explained with our small study population and high prevalence of PE. Among those 5 patients, 1 was diagnosed as PE (+), the remaining four were PE (-) (p= 0.193). We identified the sensitivity of the test as 98%, specificity 7%, positive predictive value %50, negative predictive value %80, false positivity rate %93 and false negativity rate %2. When individual parameters of PERC rule were evaluated solely for the exclusion of PE; "no leg swelling" and "no previous DVT or PE history" were found significantly negatively correlated with the diagnosis of PE (p= 0.001, r= -0.325 and p= 0.013, r= -0.214 respectively). This result was in consistence with the study of Wolf et al. that identified the unilateral leg swelling, recent surgery and history of VTE as the predictors of PE diagnosis in bivariate analysis (22). Hence, we thought that instead of providing all of the 8 criteria, some classification can be made also for the PERC, by scoring the criteria according to their power of excluding PE.
We also evaluated Wells score in this study and found a significant correlation between high Wells score (score > 6) and the PE diagnosis (p= 0.014, r= 0.230). The sensitivity of high Wells score was identified as 43%, specificity 78%, positive predictive value 66% and negative predictive value 59%. Since this is a retrospective study with low patient number we couldn't evaluated the role of combined PERC rule and Wells score or combined PERC rule and D-dimer testing in preventing excessive diagnostic testing for PE. So our results about individually assessed PERC rule and Wells score could not be attributed to general, could just be accepted as a good representative of the current status in an emergency department of a tertiary care university hospital.
But there are some studies in the literature that evaluated the PERC rule together with Wells score or revised Geneva score. In a study performed by Penalozoa A, et al., 959 patients were studied and overall PE prevelance was idenitified as 29.8%; 74 (7.7%) patients were PERC (-) and among them 4 (5.4%) patients had final diagnosis of PE (34). In this study, the combination of (-) PERC rule with low pretest probability assessed by revised Geneva Score (RGS) or gestalt clinical assessment was also evaluated. When (-) PERC rule was combined with RGS, PE prevelance was found as 6.2% and when combined with low clinical gestalt probability, PE prevelance was identified as 0%. They concluded that (-) PERC rule combined with low clinical gestalt probability seems to identify a group of patients for whom PE could easily be ruled out without additional test, but this result should be confirmed by a larger study to ensure its safety (34).
Similar to our study, Crichlow A, et al. studied the overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department (35). They studied 152 suspected PE subjects, 11.8% diagnosed with PE, which was much lower than our PE prevelance. Among those 152 suspected PE patients, 14 (9.2%) met PERC, none of whom were diagnosed with PE. A low risk Wells score (≤ 4) was assigned to 110 (72%) subjects, of whom only 38 (35%) underwent clinical D-dimer testing (elevated in 33/38). Of the 72 subjects with low risk Wells scores who did not have D-dimers performed in the emergency department, archieved research samples were negative in 16 (22%). All 21 subjects with low risk Wells scores and negative D-dimers were PE negative. CT-pulmonary angiography time (median 160 minutes) accounted for more than half of total emergency department length of study (median= 295 minutes) in this study. They concluded that, in total 9.2% and 13.8% of CT-pulmonary angiography procedures could have been avoided by use of PERC rule and Wells/D-dimer respectively (35).
As a conclusion, with our results and with the information that we obtained from the literature we can say that over investigation of PE in emergency departments still remains as an important problem. In order to prevent this, neither the clinical prediction rules (Wells score and PERC rule) nor the D-dimer test are sufficient individually. So, they must be developed further or their use and efficacy in combined form should be searched in future extensive studies.
CONFLICT of INTEREST
None declared.
REFERENCES
- The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2008;29:2276-315.
- McCaig LF. National Ambulatory Medical Care Survey. Emergency Department Summary 2000;326:1-31. Hyattsville, Maryland: National Center for Health Statistics.
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996;93:2212-45.
- Kokturk N, Demir N, Oguzulgen IK, Demirel K, Ekim N. Fever in pulmonary embolism. Blood Coagul Fibrinolysis 2005;16(5):341-7.
- Agnelli G, Becattini C. Acute pulmonary embolism. New England J Med 2010;363:266-74.
- Courtney DM, Sasser H, Pincus B, Kline JA. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation 2001;49:265-72.
- Kurkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, et al. Pulmonary embolism as a cause of cardiac arrest. Arch Intern Med 2000;160:1529-35.
- Riedel M. Emergency diagnosis of pulmonary embolism. Heart 2001;85;607-9.
- Studdert DM, Mello MM, Sage WM, DesRoches CM, Peugh J, Zapert K, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA 2005;293:2609-17.
- Taylor H. Doctors and other health professionals report that fear of malpractice have a big and mostly negative effect on clinical practice, unnecessary defensive medicine and openness to discussing medical errors. Taylor H, Leitman R (eds). Harris Interactive, Health Care News 2003;2(3):1-5.
- Kabrhel C, Matts C, McNamara M, Katz J, Ptak T. A highly sensitive ELISA D-dimer increases testing but not diagnosis of pulmonary embolism. Acad Emerg Med 2006;13:519-24.
- Goldstein NM, Kollef MH, Ward S, Gage BF. The impact of the introduction of a rapid D-dimer assay on diagnostic evaluation of suspected pulmonary embolism. Arch Intern Med 2001;161:567-71.
- Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. New England J Med 2007;357:2277-84.
- Wells PS, Anderson DR, Rodger M, Gingsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients' probability of pulmonary embolism: increasing the models utility with the Simpli RED D-dimer. J Thromb Haemost 2000;83:416-20.
- Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost 2004;2:1247-55.
- British Thoracic Society. Guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470-84.
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315.
- Turkish Thoracic Society. Pulmonary Thromboembolism Consensus Report, 2009.
- Kroegel C, Reissig A. Computed tomography imaging in pulmonary embolism-the other side of medal. Respiration 2004;71:444-7.
- Jimenez Castro D, Sueiro A, Diaz G, Escobar C, Garcia-Rull S, Picher J, et al. Prognostic significance of delays in diagnosis of pulmonary embolism. Thrombosis Resarch 2007;121:153-8.
- Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, et al. Prospective multicenter evaluation of the pulmonary embolism rule out criteria. Journal of Thrombosis and Haemostasis 2008;6:772-80.
- Wolf SJ, Mc Cubbin TR, Nordenholz KE, Naviaux NW, Haukoos JS. Assessment of the pulmonary embolism rule out criteria for evaluation of suspected pulmonary embolism in the emergency department. Am J Emergency Medicine 2008;26:181-5.
- Dachs RJ, Kulkarni D, Higgins GL 3rd. The pulmonary embolism rule out criteria rule in a community hospital ED: a retrospective study of its potential utility. Am J Emergency Medicine 2010; [epub a head of print]
- Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23.
- Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001;135:98-107.
- Hogg K, Dawson D, Kline J. Application of pulmonary embolism rule out criteria to the UK Manchester Investigation of pulmonary embolism diagnosis (MIOPED) study cohort. J Thromb Haemost 2005;3:592-3.
- Kline JA, Peterson CE, Steuerwald MT. Prospective evaluation of real time use of the pulmonary embolism rule out criteria in an academic emergency department. Academic Emergency Medicine 2010;17:1016-9.
- Lessler AL, Isserman JA, Agarwal R, Palevsky HI, Pines JM. Testing low risk patients for suspected pulmonary embolism: A decision analysis. Ann Emerg Med 2010;55:316-26.
- Carpenter CR, Keim SM, Seupaul RA, Pines JM; The Best Evidence in Emergency Medicine Investigator Group. Differentiating low risk and no risk PE patients: The PERC Score. J Emerg Med 2009;36(3):317-22.
- Pasha SM, Klok FA, Snoep JD, Mos ICM, Goekoop RJ, Rodger MA. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thrombosis Research 2010;125:e123-e7.
- Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism-revisited: a systematic review and meta-analysis. Emerg Med J 2013;30(9):701-6.
- Hugli O, Righini M, Le Gal G, Roy PM, Sanchez O, Verschuren F. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost 2011;9(2):300-4.
- Righini M, Le Gal G, Perrier A, Bounameaux H. More on: clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost 2005;3:188-9; author reply 190-1.
- Penaloza A, Verschuren F, Dambrine S, Zech F, Thys F, Roy PM. Performance of the Pulmonary Embolism Rule-out Criteria (the PERC rule) combined with low clinical probability in high prevalence population. Thromb Res 2012;129(5):e189-93.
- Crichlow A, Cuker A, Mills AM. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med 2012;19(11):1219-26.
Yaz??ma Adresi (Address for Correspondence)
Dr. M?ge AYDO?DU
Gazi ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
Be?evler, ANKARA - TURKEY
e-mail: mugeaydogdu@yahoo.com