RESEARC ARTICLE
Doi: 10.5578/tt.4740
Tuberk Toraks 2014;62(1):7-11

Pulmoner ve Ekstrapulmoner T?berk?lozlu Hastalarda
P2X7 Genindeki 1513 A/C Polimorfizmin Hastal?kla ?li?kisinin Olmamas?
Fethi Ahmet ?ZDEM?R1, Deniz EROL2, Vahit KONAR3, H?seyin Y?CE2, Ebru KARA ?ENL?4, Funda BULUT2,
Figen DEVEC?5
1 Bart?n ?niversitesi Fen Fak?ltesi, Molek?ler Biyoloji ve Genetik B?l?m?, Bart?n, T?rkiye
1 Department of Molecular Biology and Genetics, Faculty of Science, Bartin University, Bartin, Turkey
2 F?rat ?niversitesi Fen Fak?ltesi, T?bbi Genetik B?l?m?, Elaz??, T?rkiye
2 Department of Medical Genetics, Faculty of Medicine, Firat University, Elazig, Turkey
3 Sinop ?niversitesi Fen Edebiyat Fak?ltesi, Biyoloji B?l?m?, Sinop, T?rkiye
3 Department of Biology, Faculty of Science and Letters, Sinop University, Sinop, Turkey
4 Necmettin Erbakan ?niversitesi Meram T?p Fak?ltesi, T?bbi Genetik Anabilim Dal?, Konya, T?rkiye
4 Department of Medical Genetics, Meram Faculty of Medicine, Necmettin Erbakan University, Konya, Turkey
5 F?rat ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Elaz??, T?rkiye
5 Department of Pulmonary Diseases, Faculty of Medicine, Firat University, Elazig, Turkey
?ZET
Pulmoner ve Ekstrapulmoner T?berk?lozlu Hastalarda P2X7 Genindeki 1513 A/C Polimorfizmin Hastal?kla ?li?kisinin Olmamas?
Giri?: Latent, pulmoner ve ekstrapulmoner ?eklinde farkl? klinik formlar? olan t?berk?loz, d?nya genelinde ?l?me sebebiyet veren hastal?klar?n en ?nemlilerinden biridir. Aday genlerle yap?lan ?al??malarda yayg?n polimorfizmlerin t?berk?lozun geli?mesinde etkili olabilece?i sonucuna var?lm??t?r. Bu ?al??mada P2X7 genindeki 1513 A/C polimorfizminin t?berk?lozun etyopatogenezisindeki rol?n?n ortaya ??kar?lmas? ama?lanm??t?r.
Materyal ve Metod: Bu ?al??ma 160 t?berk?lozlu (71 pulmoner t?berk?lozlu ve 89 ekstrapulmoner t?berk?lozlu) 160 da sa?l?kl? bireyi kapsamaktad?r. Genomik DNA izolasyonu ger?ekle?tirildikten sonra P2X7 genindeki 1513 A/C polimorfizmi PCR-RFLP metoduyla genotiplendirildi.
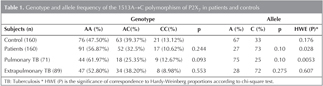
Bulgular: AA genotipinin frekans? kontrol grubunda %47.5, hastalarda %56.87, AC genotipinin frekans? kontrol grubunda %39.37, hastalarda %32.5, CC genotipinin frekans? kontrol grubunda %13.12, hastalarda %10.62 olarak saptand?. Hastalar ile kontrol grubunun allel ve genotip frekanslar? aras?nda anlaml? bir farkl?l?k bulunamad?.
Sonu?: Sonu?lar?m?z T?rkiye'nin do?u b?lgesinde P2X7 genindeki, 1513 A/C polimorfizminin pulmoner ve ekstrapulmoner t?berk?loz hastal???yla ili?kisinin olmad???n? g?sterdi.
Anahtar kelimeler: T?berk?loz, P2X7, polimorfizm
SUMMARY
Lack of Association of 1513 A/C Polymorphism in P2X7 Gene with Susceptibility to Pulmonary and Extrapulmonary Tuberculosis
Introduction: Tuberculosis, is one of the a leading causes of death worldwide, is characterized by different clinical forms including: latent, localized pulmonary infection and extrapulmonary tuberculosis. Candidate gene association studies have implicated common polymophisms in genes that may influence the development of tuberculosis. This study, aimed to elucidate the role of P2X7 gene in 1513A/C polymorphism? the etiopathogenesis of tuberculosis.
Materials and Methods: The study included 160 patients with tuberculosis (71 pulmonary and 89 extrapulmonary tuberculosis) and 160 healthy controls. Genomic DNA was isolated and 1513A/C polymorphism in P2X7 gene was genotyped by PCR-RFLP method.
Results: Frequency of P2X7 AA genotype was 47.5% in controls and 56.87% in patients, AC frequency was 39.37% controls and 32.5% in patients, CC genotype was 13.12% in controls and 10.62% in patients. No significant difference in allele and genotype frequencies (1513A/C polymorphism) between tuberculosis patients and controls was found.
Conclusion: The results suggest that 1513A/C polymorphism of P2X7 gene is not associated with pulmonary and extrapulmonary tuberculosis in the Eastern Turkey.
Key words: Tuberculosis, P2X7, polymorphism
INTRODUCTION
Tuberculosis is a major cause of morbidity and mortality worldwide, especially in Asia and Africa. Genetic variability, combined with environmental factors, are shown to contribute to the risk of developing active tuberculosis (1). Some patients have identifiable risk factors such as diabetes mellitus, malnutrition, human immunodeficiency virus (HIV) infection or immunosuppressive therapy; however many patients exhibit none of these clinical risk factors (2).
Some genetic variations such as Mendelian-inherited mutations in the genes encoding interferon-gamma (IFN-γ), interleukin-12, and signal transducers are rare and are associated with severe mycobacterial infection. Recently some researchers have reported that polymorphism in IFN-γ and MCP-1 genes were associated with an increased risk of developing active tuberculosis in some Tunisian patients (3). Other genetic variations such as polymorphisms in the genes encoding human leukocyte antigen (HLA), P2X7 receptor, the solute carrier family 11 member a1 protein (SLC11A1, formerly known as NRAMP1), and vitamin D3 receptor (VDR), which occur more commonly, are considered to account for the susceptibility of the general population to tuberculosis (4,5).
Human P2X7, which encodes the P2X7 receptor, has been cloned and mapped to the human chromosome 12q24 and linked to tuberculosis susceptibility (6). P2X7 is highly polymorphic and several single nucleotide polymorphisms (SNPs) that lead to the loss of receptor function have been identified (7,8). The most common is the 1513A→C polymorphism because glutamic acid at position 496 changes to alanine. The function of the P2X7 receptor in macrophages from subjects homozygous for the 1513C allele is ablated, and the function of the P2X7 receptor in macrophages from heterozygous subjects is significantly impaired.
The aim of this study was to determine the genotype and allelic distribution and possible link to susceptibility to tuberculosis of P2X7 1513A→C polymorphism in the Eastern Turkey.
Materials and Methods
Patients and Control Group
The study was approved by the ethics committee of Firat University Medical Faculty. A total of 160 patients with active tuberculosis (71 pulmonary tuberculosis, 89 extrapulmonary tuberculosis patients; with mean age 37.43 ? 14.58 years) were recruited from those who were treated and followed up in the Pulmonary Diseases Department of the Firat University Hospital, Turkey. As healthy controls, age, sex and origin-matched 160 unrelated selected healthy subjects (mean age 39.27 ? 13.84 years).
Patients were recruited between September 2009 and May 2011 and were selected after confirmation of the infection according to the criteria defined by the American Thoracic Society (9). The diagnosis of active pulmonary tuberculosis was based on clinical examination and the presence of the Mycobacterium tuberculosis strain in sputum smears and cultures on Lowenstein-Jensen and Coletsos media. Active extrapulmonary tuberculosis was identified by histological examination (granulomatous formations) of data and confirmed by conventional bacteriological methods.
Genotype Analysis
Peripheral blood samples were drawn from all of the participants, and were taken into tubes containing ethylenediamine tetraacetate (EDTA) and DNA was extracted and stored at -20?C until analysis of the P2X7 polymorphism.
Genomic DNA was prepared from 300 ?L of fresh blood peripheral blood mononuclear cells using a Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI) according to the manufacturer's recommendations. Afterwards the samples were quantified using a Nanodrop spectrophotometer (UV-Visible NanoDrop 1000, Thermo Fisher Scientific Inc.) and standardized to 50 ng ?L-1. Aliquots were stored at -20?C for further genotyping.
The 1513A→C SNP was genotyped by PCR-restriction fragment length polymorphism (RFLP) with the following primers: 5'-AGA CCT GCG ATG GAC TTC ACA G-3' (forward) and 5'-AGC GCC AGC AAG GG CTC-3' (reverse) (10). The PCR conditions were: initial denaturation at 95?C for 5 minutes; 36 cycles of 95?C for 30 seconds, 36 cycles of 66.3?C for 30 seconds, 36 cycles of 72?C for 40 seconds, and final elongation at 72?C for 7 minutes. The PCR products were digested at 37?C for 4 hours with 5.0 U of HaeII (Promega). The digested products were run on a 3% agarose gel and visualized with 10 ng/mL ethidium bromide.
Statistical Analysis
Statistical analyses were carried out using SPSS software version 16 (SPSS Inc. Chicago IL USA). The genotype distribution was tested for Hardy-weinberg equilibrium using chi-square test in tuberculosis patients and controls. The distributions of P2X7 polymorphism between tuberculosis patients and healthy controls were compared using the Fisher's exact test. p< 0.05 was considered significant.
Results and Discussion
Genotypes and alleles frequencies of the 1513A→C polymorphism of P2X7 gene in tuberculosis patients and controls are shown in the Table 1.
The frequency of the 1513A allele in the tuberculosis patients was 0.27, where as that of 1513C was 0.73, and no significant differences were noted in comparison with the frequencies in the case of the control subjects (p> 0.05, Table 1). Analysis of genotypic distribution using chi-square test revealed no significant difference between the two groups (p> 0.05, Table 1). Moreover, no significant associations were found between the genotypic or the allelic distributions and pulmonary or extrapulmonary tuberculosis.
This study was undertaken to gain insight into the role of the human P2X7 receptor gene in the susceptibility to tuberculosis in the Eastern Turkey. Areas of inflamed tissue such as tuberculosis granulomata contain numerous monocytes, macrophages and high local concentrations of ATP (released from dying and activated cells) and pro-inflammatory cytokines (11). It is possible that the ATP- linked P2X7 effector pathway may contribute to host immunity of M. tuberculosis.
There is substantial evidence that show host genetic factors are important in determining susceptibility to mycobacteria (12,13). To the best of our knowledge, this is the first study investigating the association between P2X7 genetic polymorphisms and the susceptibility to pulmonary and extrapulmonary tuberculosis in the Eastern Turkey.
Several SNPs in the P2X7 gene that affect the function of this receptor have been described. The more relevant SNPs of P2X7 reported correspond to the 1513A/C that affects the carboxy terminal tail and leads to a loss of receptor function as assessed by ATP induced Ca2+ ethidium bromide influx and IL-1β release (14,15,16,17,18). Previous studies have shown that macrophages from individuals homozygous for the 1513C loss of function allele have complete loss of receptor function while individual heterozygous for this polymorphism still maintain 50% function to kill intracellular parasites such as M. tuberculosis or T. gondii after exposure to ATP compared with macrophages from individuals with the 1513A wild type allele (17,18).
This study indicates that there is no risk between 1513A/C polymorphism in the P2X7 gene and tuberculosis disease in the Eastern Turkey. These results are in agreement with previously reported data in an Australian Vietnamese population and a Chinese Han population (17,19). It was reported in other studies in Mexicans, Russians and Tunisians an association was found between the 1513A/C polymorphism and susceptibility to active tuberculosis (10,20,21).
The heterogenity of the results reported by these studies could be related to one or more of the following parameters: (a) the ethnic origin of patients. For example, it has been reported that ethnic-specific genetic variations may influence host immunity to tuberculosis, causing different tuberculosis susceptibilities (22). In this setting, among control subjects, the prevalence of the 1513C loss of function allele was 7.6% in a Gambian population, 13% in a Russian Slavic population, 17% in a Tunisian population, 19% in a Mexican population, 20.6% in a Vietnamese population and 33% in Turkish population (the present study); (b) Sample size and study design which may affect statistical calculations; (c) As genetic susceptibility to tuberculosis is polygenic, the other described functional SNPs occurring in the P2X7 gene may be associated with susceptibility to active tuberculosis (10,17,20,21,22,23). Further studies are needed to investigate whether this functional polymorphism is associated with a risk of developing active tuberculosis.
Our results indicate that the 1513A/C polymorphism of P2X7 are not associated with an increased susceptibility to M. tuberculosis infection in this population. Because host susceptibility to tuberculosis is likely to be under polygenic control, and the risks attributable to each polymorphism is modest, the precise mechanism(s) of underlying susceptibility or protection, as well as its possible clinical relevance, remains an interesting topic to be explored further.
Acknowledgement
This study was sponsored by Scientific Research Projects Unit (FUBAP) of the Firat University (Project no: FF.10.01).
CONFLICT of INTEREST
None declared.
REFERENCES
- Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet 2001;2:967-77.
- Barnes DS. Historical perspectives on the etiology of tuberculosis. Microbes Infect 2000;2:431-40.
- Ben-Selma W, Harizi H, Bougmiza I, Hannachi N, Kahla IB, Zaieni & Boukadida J. Interferon gamma +874 T/A polymorphism is associated with susceptibility to active pulmonary tuberculosis development in Tunisian patients. DNA Cell Biol 2011;2011:379-87. DOI: 10.1089/dna.2010.1157.
- D?ffinger R, Dupuis S, Picard C, Fieschi C, Feinberg J, Barcenas- Morales G & Casanova JL. Inherited disorders of IL-12- and IFN gamma-mediated immunity: a molecular genetics update. Mol Immunol 2001;38:903-9.
- Bellamy R. Susceptibility to mycobacterial infections: the importance of host genetics. Genes Immun 2003;4:4-11.
- Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA & Antonarakis SE. Gene structure and chromosomal localization of the human P2X7 receptor. Receptor Channel 1998;5:347-54.
- Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS & Britton WJ. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis 2005;192:149-55.
- Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 2006;281:2079-86.
- American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Resp Crit Care 2000;161:1376-95.
- Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, Portales-Cervantes L, Flores-Meraz V, Baranda L, et al. P2X7 and NRAMP1/SLC11 A1 gene polymorphism in Mexican metsizi patients with pulmonary tuberculosis. Clin Exp Immunol 2007;148:469-77.
- Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro Express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J Cell Sci 1999;112:297-305.
- Marquet S, Schurr E. Genetics of susceptibility to infectious diseases: tuberculosis and leprosy as examples. Drug Metab Dispos 2001;29:479-83.
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 2002;20:581-620.
- Gu BJ, Zhang W, Worthington RA, Sluyter R, DAo-Ung P, Petrou S, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 2001;276:11135-42.
- Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss of function polymorphism in the human P2X7 receptor abolishes ATP mediated killing of mycobacteria. J Immunol 2003;171:5442-6.
- Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5'-triphosphate induced interleukin-18 release from human monocytes. Genes Immun 2004;5:588-91.
- Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Resp Crit Care 2007;175:360-6.
- Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol 2010;184:7040-6.
- Xiao J, Sun L, Jiao W, Li Z, Zhao S, Li H, et al. Lack of association between polymorphisms in the P2X7 gene and tuberculosis in a Chinese Han population. FEMS Immunol Med Mic 2009;55:107-11.
- Mokrousov I, Sapozhnikova N, Narvskaya O. Mycobac-terium tuberculosis coexistence with humans: making an imprint on the macrophage P2X7 receptor gene? J Med Microbiol 2008;57:581-4.
- Ben-Selma W, Ben-Kahla I, Boukadida J, Harizi H. Contribution of the P2X7 1513A/C loss of function polymorphism to extrapulmonary tuberculosis susceptibility in Tunisian populations. FEMS Immunol Med Mic: 2011:1-8.DOI:10.1111/j.1574-695X.2011.00824.x.
- M?ller M, Hoal EG. Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberculosis 2010;90:71-83.
- Li CM, Campbell SJ, Kumararatne DS, Bellamy R, Ruwende C, McAdam KP, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis 2002;186:1458-62.
Yaz??ma Adresi (Address for Correspondence)
Dr. Fethi Ahmet ?ZDEM?R
Bart?n ?niversitesi Fen Fak?ltesi,
Molek?ler Biyoloji ve Genetik B?l?m?,
BARTIN - TURKEY
e-mail: ozdemirfethiahmet23@yahoo.com