KOAH ve akci?er kanserinde ya?la ili?kili olarak GST polimorfizmi kar??la?t?rmal? ?al??ma
Rajni KANT SHUKLA1, Surya KANT2, Balraj MITTAL3, Sandeep BHATTACHARYA1
1 King George T?p Fak?ltesi, Fizyoloji B?l?m?, Lucknow,
2 King George T?p Fak?ltesi, G???s Hastal?klar? B?l?m?, Lucknow,
3 Sanjay Gandhi Y?ksek Lisans T?bbi Bilimler Enstit?s?, T?bbi Genetik B?l?m?, Lucknow.
?ZET
KOAH ve akci?er kanserinde ya?la ili?kili olarak GST polimorfizmi kar??la?t?rmal? ?al??ma
Giri?: Glutatyon-S-transferaz ksenobiyotik bile?enlerinin detoksifikasyonunda g?rev al?r. GST gen polimorfizmi solunum sistemi hastal?klar?nda olas? bir de?i?tirici olarak kabul edilmektedir. KOAH akci?erlerin yava? ilerleyen kronik inflamatuvar bir hastal???d?r. ?o?unlukla ya?l?larda g?r?l?r ve baz? ?al??malarda kanser i?in ya? ba?l?ca risk fakt?r? olarak varsay?lmaktad?r. Bu ?al??mada; Kuzey Hindistan'da KOAH ve akci?er kanseri hastalar?nda ya?la metabolik fazlar?nda etkili iki gen olan GSTT1 ve GSTM1 gen polimorfizminin ara?t?r?lmas? ama?lanm??t?r.
Materyal ve Metod: ?al??maya akci?er kanseri, KOAH hastalar? ve kontrol grubu al?nd?. Hastalardan spirometrik ve histopatolojik de?erlendirme sonras?nda periferik kan ?rne?i al?nd?. T?m genotiplendirme PCR-RFLP y?ntemiyle yap?ld?. Devaml? de?i?kenlerin ortalama de?erlerinin kar??la?t?r?lmas? ba??ms?z ki-kare testiyle yap?ld?. ?statistiksel anlaml? farkl?l?k hastalarda ve kontrol grubunda genotip s?kl?klar?ndaki farkl?l?k ki-kare testiyle de?erlendirildi.
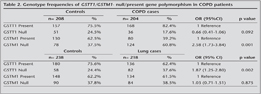
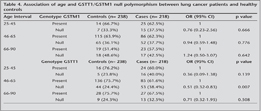
Bulgular: GSTM1 null polimorfizmi sa?l?kl? kontrollerle kar??la?t?r?ld???nda KOAH'l? hastalarda anlaml? olarak y?ksek bulundu (OR= 2.08; 95%; CI= 1.40-3.09; p= 0.0001), hastalar ve kontrol grubu aras?nda GSTT1 null polimorfizmi s?kl???nda fark yoktu (OR= 1.87; 95%; CI= 1.25-2.80; p= 0.002). GSTM1 null akci?er kanseriyle ili?kili bulunmad?. Alt grup analizinde GSTM1 null polimorfizmi 46-65 ya? grubundaki KOAH'l? hastalarda kontrol grubuyla kar??la?t?r?ld???nda anlaml? bulundu (62.2%/37.8%, OR= 3.20; 95%; CI= 1.97-5.18; p= 0.001), akci?er kanserli hastalar ile kontrol aras?nda ayn? ya? grubunda fark yoktu (55.6%/44.4% OR= 1.07; 95% CI= 0.68-1.69; p= 0.774).
Sonu?: GSTM1 null genotipinin etkileri KOAH ile g??l? bir ili?ki g?stermektedir. GSTT1 null genotipi ise akci?er kanserli hastalarda etkilidir. GSTM1 null genotipi ?zellikle orta ya? grubunda artm?? KOAH riskiyle ili?kili bulundu.
Anahtar Kelimeler: Glutatyon-S transferaz, akci?er kanseri, kronik obstr?ktif akci?er hastal???.
SUMMARY
Comparative study of GST polymorphism in relation to age in COPD and lung cancer
Rajni KANT SHUKLA1, Surya KANT2, Balraj MITTAL3, Sandeep BHATTACHARYA1
1 Department of Physiology, King George's Medical University, Lucknow, India,
2 Department of Pulmonary Medicine, King George Medical University, Lucknow, India,
3 Department of Medical Genetics, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Introduction: Glutathione-S-transferase is involved in detoxification of xenobiotic compounds. GSTs gene polymorphisms have been considered as a potential modifier for the respiratory disease. COPD is a chronic inflammatory disease of the lungs, which progresses very slowly and the majority of patients are therefore elderly, and few study suggested that age is major risk factor for cancer. Whether age in metabolism of phases 2 enzyme gene polymorphisms GSTT1 and GSTM1 in Northern Indian COPD and lung cancer patients.
Materials and Methods: We have enrolled lung cancers, COPD patients and for the comparison we enrolled controls. Peripheral blood of COPD and lung cancer patients was taken after spirometry evaluation and histopathology. All genotyping were done by PCR-RFLP method. Independent sample t-test was performed to compare the mean values of continuous data. Statistical significance of differences in genotype frequencies between patients and controls was estimated by the chi-square two test.
Results: GSTM1 null polymorphism was found to be significantly higher in COPD patients as compared with healthy controls (OR= 2.08; 95%; CI= 1.40-3.09; p= 0.0001), but there were no significant differences polymorphisms of GSTT1 null patients and healthy controls. In lung cancer GSTT1 null was found significantly associated (OR= 1.87; 95%; CI= 1.25-2.80; p= 0.002) however GSTM1 null was not associated with lung cancer patients. In subgroup analysis, we found GSTM1 Null significantly associated between age 46-65 years in COPD patients and healthy controls (62.2%/37.8%, OR= 3.20; 95%; CI= 1.97-5.18; p= 0.001), In lung cancer and controls (55.6%/44.4% OR= 1.07; 95% CI= 0.68-1.69; p= 0.774).
Conclusion:? The effects of the GSTM1 null genotype seemed strong association with COPD and GSTT1 null genotype with lung cancer patients. GSTM1 null genotype is associated with an increased risk of COPD, especially in middle age.
Key Words: Glutathione-S-transferase, lung cancer, chronic obstructive pulmonary disease.
Tuberk Toraks 2013; 61(4): 275-282 • doi:10.5578/tt.6252
Geli? Tarihi/Received: 04/07/2013 • Kabul Edili? Tarihi/Accepted: 24/10/2013
Introduction
Respiratory system undergoes various structural, physiological, and immunological changes with age. Chronic obstructive pulmonary disease (COPD) now affects 10% of the world population over the age of 40 years, and the burden of disease is particularly high in developing countries (1). Not only aging is responsible for the development of COPD it also a role in the development of cancer also (2). Most of Xenobiotic compounds are firstly metabolized by the lung, because lung is the foremost site for xenobiotic metabolism. Lung conspicuously is unruffled? more than 40 different cell types and these different cell types have different levels of metabolic competence (3). In lung tissue alveolar macrophages and bronchial epithelial cells are part of > 40 different cell types and their biological functions is differ from the others cells and it is well define alveolar macrophages cell is the important defence mechanism from the tobacco smoke particles for the clarence of airways (4,5). Lung epithelium is metabolite the tobacco smoke toxic component in phase I and phase II enzymes [like Cyp P450 and Glutathione-S-Transferase (GSTs)] (6,7).
Lung cancer is the most common malignancy and the leading cause of death in men worldwide (8). Tobacco smoking is the strongest known risk factor for developing lung cancer. GST enzymes are involved in the detoxification of potential carcinogens present in tobacco smoke, and polymorphisms in GST genes have been intensely studied as possible modulators of risk for lung cancer. GSTs are a multigene family of phase II enzymes that detoxify electrophilic metabolites through conjugation with reduced glutathione followed by excretion from the body (9).
COPD is a cluster of heterogenic disorders, characterized by expiratory flow limitation (10). Cigarette smoke is the most commonly encountered risk factor for this disease, as well as in the development of a COPD co-morbidity, the lung cancer the deadliest cancer in men and women around the world (11,12,13). Most of the studies have shown that smokers who develop COPD have a higher risk to develop lung cancer than no smokers (14,15).
Theoretically early onset of lung cancer may have well built genetic component in its pathology than is the case for lung cancer at older ages, and many studies have reported GST gene polymorphism have strongly associated with cancer risk at younger ages (16,17,18).
On the basis of above literature we proposed that whether age in metabolism of phase 2 enzyme gene polymorphisms GSTT1 and GSTM1 in Northern Indian COPD and lung cancer.
MATERIALS and METHODS
All the patients with COPD were diagnosed according to GOLD guideline and? recruited Outpatient Department of Pulmonary Medicine, King George's Medical University Lucknow, (Erstwhile Chhatrapati Shahuji Maharaj Medical University), 408 subjects (204 COPD patients and 208 Healthy controls) (19). The lung function of the subjects was assessed as FEV1 % predicted, that is, FEV1 adjusted for age, height, and sex, and airflow limitation with post bronchodilator FEV1 < 80% predicted, FEV1/ forced vital capacity (FVC) < 70% and improvement in FEV1< 12% or 200 mL after inhalation of a bronchodilator (20). They were all smokers and non-smoker.
Corresponding to the COPD group, 179 males and 25 females (mean age 57.83 ? 10.58 years) and in healthy controls 166 males and 42 females (mean age 56.35 ? 8.12 years) were included. Exclusion criteria were respiratory disorders other than COPD, such as interstitial lung disease, any type of malignancy, previous history of asthma, tuberculosis were excluded. In case of lung cancer patients 456 (218 lung cancer cases and 238 healthy controls) were recruited from the Department of Pulmonary Medicine, King George's Medical University, Lucknow (Erstwhile C.S.M.M University), India. All cases were newly diagnosed and previously treated patients were examined by histological.
All controls age-sex matched were recruited without a previous medical history of respiratory disorders and without any present respiratory symptoms, seen by their practitioner. The study was approved by the ethical committee of institution King George's Medical University (Erstwhile CSMMU) and subject gave their written informed consent.???
DNA Extraction
Blood (5 mL) for genotyping was drawn into an EDTA- tube and genomic DNA was extracted from peripheral blood leukocytes pellet using the standard salting-out method (21).
Genotype Analysis
Null alleles of GSTM1 and GSTT1 were determined by using multiplex polymerase chain reaction (PCR) with the CYP1A1 gene as an internal positive control (22). Briefly, a 215-bp region between exons 4 and 5 of the GSTM1 gene and 480-bp products for GSTT1 were amplified along with a 312-bp size product of CYP1A1. The PCR products were electrophoresed on a 2% agarose gel. The absence of 480 and 215 bp bands indicated homozygous null genotypes of GSTM1 and GSTT1, respectively.
Statistical Analysis
Descriptive statistics of patients and controls were presented as mean and standard deviations for continuous measures whereas frequencies and percentages were used for categorical measures. The independent sample t-test was performed to compare the mean values of continuous data. The statistical significance of differences in genotype frequencies between patients and controls was estimated by the chi-square test. The clinical parameters were expressed as Mean ? SD. Odds ratio (OR), 95% confidence interval (CI) and a p value of genotype data were adjusted for confounding factors such as age, sex and pack years of smoking. A p value of ≤ 0. 05 was considered statistically significant.
Results
The mean age of healthy subjects (controls) and Lung cancer patients was 56.15 ? 7.84 and 56.14 ? 11.91 years, respectively (t-test p value= not significant). Cancer was highly prevalent in males (189 out of 218; 86.7%) than in females (29 out of 218; 13.3%). Demographic characteristics were shown in Table 1. In patients with lung cancer most of the cases were with squamous cell carcinoma (54.1%). In case of smoking habit, 58.7% smokers, 9.6% ex-smokers and 31.7% non-smokers in lung cancer cases. Mean pack years is 13.95 ? 7.93 (years), as compared with controls 72.3% were non-smokers, 13.4% ex-smoker and 14.3% smokers.
Association of Genetic Variations with Susceptibility to COPD and Lung Cancer
There were consistent patterns of elevated risk associated with the null GSTM1 Null genotype in COPD patients as compared with healthy controls (60.8% vs. 37.5%, OR= 2.58; 95% CI, 1.73-3.84; p= 0. 001), and in case of? lung cancer? frequency of the null GSTT1 genotype was higher in comparison with healthy controls [37.6% vs. 24.4%, OR= 1.87, 95% CI= 1.25-2.80, p= 0. 002) (Table 2)].
Association of Age and GSTT1/GSTM1 Null Polymorphism Between COPD and Healthy Controls
GSTM1 null genotype was being significantly higher in the age group of 46-65 years. In comparison with control (62.2% vs. 37.8%, OR= 3. 20, 95%CI= 1.97-5.18, p= 0.001), but GSTT1 null genotype was no significant association with all the age groups (Table 3).
Association of Age and GSTT1/GSTM1 Null Polymorphism Between Lung Cancer and Healthy Controls
Instead of, I did not find any significant association of GSTs gene polymorphism with the lung cancer patients of any age groups (Table 4).
Discussion
GSTT1 and GSTM1 catalyze the detoxification of many carcinogens and anti-neoplastic drugs, and they can also deactivate selected xenobiotics to form genotoxic products in this we supposed that GSTM1 and GSTT1 null polymorphism were associated with age in the metabolism of xenobiotic compound as a risk for the development of? COPD disease as well as lung cancer (23,24,25).
Our study results show that GSTT1 null polymorphism were associated with the lung cancer Northern Indian population (p= 0.002) as compared with the controls (37.6% vs. 24.4%). In case of COPD patients GSTM1 null polymorphism was associated with the COPD Northern Indian population (p= 0.001) as compared with the controls (60.8% vs. 37.5%).
Prevalence of null genotypes in GSTM1 and/or both GSTT1 genes in our population is comparable with that found in other Indian studies (26,27). As a result of deletions in one or both of these loci and, consequently, less detoxification of xenobiotic toxic substances, an individual may become susceptible to diseases produced by toxic substances present in the environment; hence, finding a positive correlation raises the possibility that the two enzymes are working in tandem rather than in a complementary way.
Tobacco smoke constitutes a complex mixture of thousands of toxicants, some of which require tissue-specific activation to become genotoxic carcinogens and others become metabolically activated poisons (28,29). For smokers, the risk of developing lung cancer has been shown to be, at least in part, dependent on the pulmonary metabolism of smoke constituents (30). Particular evidence stems from studies with smokers in which genetic polymorphisms of certain xenobiotic metabolizing enzymes were linked to the development of lung cancer (31,32).
There is still a lack of fundamental lack of knowledge on cellular, molecular and genetic causes of lung cancer as well as COPD. The best of our knowledge all the current therapy are inadequate because no treatments reduce the disease progress or mortality.
Senescence or aging is defined as the progressive decline of homeostasis that occurs after the reproductive phase of life is complete, leading to an increasing risk of disease or death. Kirkwood? has advanced the concept of ?disposable soma,? in which aging, rather than being programmed and determined by selected genes, results from the stochastic interaction between injury and repair, as the result of the energy devoted by an individual to maintain organ integrity and protect DNA against oxidative injury (33).
GSTM1 Null genotype have 3 fold risk? in middle age group control vs. cases (36.2% vs. 64.5%), 3.25, p= 0.001 and? GSTT1 null genotype have? risk in middle age group? control and cases (24.4% vs. 38.4%), 0.51, p= 0.007.
Aging is a complex process of functional decline and increased disease risk that has been resulted from the accumulation of DNA mutations. A series of mutations in key regulatory genes are the main reason of cancer induction. Genes involved in the control of genome instability, DNA repair, cell cycle regulation, apoptosis, and such processes as xenobiotics detoxification, are the main candidate for aging and carcinogenesis. Mutations affecting the functions of these genes cause the range of abnormalities.
Polymorphic variants of their DNA sequences can modify the functions of genes and predispose to the disease development in combination with other genetic and environmental factors. The frequency of alleles of polymorphic genes is determined by natural selection. Many factors affect the genomic polymorphism spectrum in populations, such as geographical location, ethnicity, type of diet, habits, etc.
Numerous of molecular epidemiological studies have been devoted to finding biomarkers of age-related diseases. However, the revealing of reliable association between polymorphic allele variants and susceptibility to disease depends on allele frequency in the population. The high frequency of allele facilitates the identification of the association with high probability. In the case of rare allele the detection of association is more complicated, and it requires an increase of sample size (34).
A study was conducted by Fletcher and Peto demonstrated that death and disability from COPD were related to an accelerated decline in lung function with time, with a loss of 50 to 100 mL in FEV1 per year, but even in healthy volunteers there is a loss of 20 mL per year with aging (35). Janssens and coworkers demonstrated that physiologic aging of the lung is associated with dilatation of alveoli with an enlargement of airspaces and a decrease in gas exchange surface area, together with a loss of supporting tissue for peripheral airways (senile emphysema), resulting in decreased static elastic recoil of the lung and increased residual volume and functional residual capacity (36).
The GSTM1 null genotype has been suggested a risk for the development of COPD. But we found that GSTT1 null genotype was not associated with the COPD patients. Our study results were also suggested that middle age COPD has stronger genetic component than COPD at an older age. However such genetic association with age can be inferred from lung cancer Northern Indian population.
Acknowledgment
The study was supported by a research grant from Council of Science and Technology U.P., Lucknow (UPCST/SERPD-D-3404) and Indian Council of Medical Research, New Delhi.
CONFLICT of INTEREST
None declared.
REFERENCES
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741-50.
- Chang MY, Boulden J, Katz JB, Wang L, Meyer TJ, Soler AP, et al. Bin1 ablation increases susceptibility to cancer during aging, particularly lung cancer. Cancer Res 2007; 67: 7605-12.
- Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol 2003; 43: 149-73.
- Hukkanen J, Pelkonen O, Hakkola J, Raunio H. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Crit Rev Toxicol 2002; 32: 391-411.
- Drath DB, Karnovsky ML, Huber GL. Tobacco smoke. Effects on pulmonary host defense. Inflammation 1979; 3: 281-8.
- Crawford EL, Khuder SA, Durham SJ, Frampton M, Utell M, Thilly WG, et al. Normal bronchial epithelial cell expression of glutathione transferase P1, glutathione transferase M3, and glutathione peroxidase is low in subjects with bronchogenic carcinoma. Cancer Res 2000: 60: 1609-18.
- Han W, Pentecost BT, Pietropaolo RL, Fasco MJ, Spivack SD.? Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol Carcinog 2005; 44: 202-11.
- Mettlin C. Global breast cancer mortality statistics. CA Cancer J Clin 1999; 49: 138-44.
- Landi S. Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 2000; 463: 247-83.
- Broekhuizen BD, Sachs AP, Hoes AW, Verheij TJ, Moons KG.? Diagnostic management of chronic obstructive pulmonary disease. Neth J Med 2012; 70: 6-11.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532-55.
- Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer 2002; 97: 72-81.
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. CA Cancer J Clin 2001; 51: 15-36.
- Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2002; 62: 51-6.
- Mina N, Soubani AO, Cote ML, Suwan T, Wenzlaff AS, Jhajhria S, et al. The relationship between chronic obstructive pulmonary disease and lung cancer in African American patients. Clin Lung Cancer 2012; 13: 149-56.
- Nascimento H, Coy CS, Teori MT, Boin IF, Goes JR, Costa FF, et al. Possible influence of glutathione S-transferase GSTT1 null genotype on age of onset of sporadic colorectal adenocarcinoma. Dis Colon Rectum 2003; 46: 510-5.
- Kote-Jarai Z, Easton D, Edwards SM, Jefferies S, Durocher F, Jackson RA, et al. Relationship between glutathione S-transferase M1, P1 and T1 polymorphisms and early onset prostate cancer. Pharmacogenetics 2001; 11: 325-30.
- Taioli E, Gaspari L, Benhamou S, Boffetta P, Brockmoller J, Butkiewicz D, et al. Polymorphisms in CYP1A1, GSTM1, GSTT1 and lung cancer below the age of 45 years. Int J Epidemiol 2003; 32: 60-3.
- GOLD. Global strategy for th diagnosis, managment and prevention of chronic obstrutive pulmonary disease. Global Initiative for Chroinc Obstrutive Lung Disease (GOLD) 2009.
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123: 659-64.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1998; 16: 1215.
- Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev 2000; 9: 73-80.
- Keen JH, Jakoby WB. Glutathione transferases. Catalysis of nucleophilic reactions of glutathione. J Biol Chem 1978; 253: 5654-7.
- Hallier E, Schroder KR, Asmuth K, Dommermuth A, Aust B, Goergens HW. Metabolism of dichloromethane (methylene chloride) to formaldehyde in human erythrocytes: influence of polymorphism of glutathione transferase theta (GSTT1-1). Arch Toxicol 1994; 68: 423-7.
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005; 45: 51-88.
- Naveen AT, Adithan C, Padmaja N, Shashindran CH, Abraham BK, Satyanarayanamoorthy K, et al. Glutathione S-transferase M1 and T1 null genotype distribution in South Indians. Eur J Clin Pharmacol 2004; 60: 403-6.
- Mishra DK, Kumar A, Srivastava DS, Mittal RD. Allelic variation of GSTT1, GSTM1 and GSTP1 genes in North Indian population. Asian Pac J Cancer Prev 2004; 5: 362-5.
- Smith GB, Bend JR, Bedard LL, Reid KR, Petsikas D, Massey TE. Biotransformation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in peripheral human lung microsomes. Drug Metab Dispos 2003; 31: 1134-1141.
- Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 1992; 13: 1789-94.
- Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis? 2001; 22: 1903-30.
- Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev 2000; 9: 3-28.
- London SJ, Idle JR, Daly AK, Coetzee GA. Genetic variation of CYP2A6, smoking, and risk of cancer. Lancet 1999; 353: 898-99.
- Kirkwood TB. Understanding the odd science of aging. Cell 2005; 120: 437-47.
- Djansugurova LB, Perfilyeva AV, Zhunusova GS, Djantaeva KB, Iksan OA, Khussainova EM. The determination of genetic markers of age-related cancer pathologies in populations from Kazakhstan. Front Genet 2013: 4: 70.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645-8.
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 1999; 13: 197-205.
Yaz??ma Adresi (Address for Correspondence):
Dr. Sandeep BHATTACHARYA,
King George T?p Fak?ltesi,
Fizyoloji B?l?m?, LUCKNOW- INDIA
e-mail: dr.sabhattacharya@gmail.com