Bronkopulmoner displazi tedavisinde postnatal oral hidrokortizon tedavisi:
G?zlemsel bir ?al??ma
Banu
MUTLU, Gonca SANDAL, G?zde KANMAZ, Zeynep ERASLAN, ?mer ERDEVE, ?erife Suna O?UZ,
U?ur D?LMEN
Ankara
Dr. Zekai Tahir Burak Kad?n Sa?l??? E?itim ve Ara?t?rma Hastanesi,
Yenido?an
Yo?un Bak?m ?nitesi, Ankara.
?ZET
Bronkopulmoner displazi tedavisinde postnatal oral hidrokortizon tedavisi: G?zlemsel bir ?al??ma
Giri?:?al??mam?z?n amac?, bronkopulmoner displazi tedavisi i?in hidrokortizonun d???k dozda bile etkili ve g?venli bir alternatif tedavi oldu?unu belirlemektir.
Materyal ve Metod: Bu prospektif pilot ?al??ma Dr. Zekai Tahir Burak Kad?n Sa?l??? ve Hastal?klar? E?itim ve Ara?t?rma Hastanesindeki ???nc? d?zey yenido?an yo?un bak?m ?nitesinde yap?ld?. Yakla??k olarak ?? hafta ve ?st?nde? ventilat?r ba??ml?l???? olan (kurtarma tedavisi olarak tan?mland?) veya postkonsepsiyonel 36. haftada herhangi bir infeksiyon kan?t? olmadan oksijen ba??ml?l??? devam eden (bronkopulmoner displazi tedavisi olarak tan?mland?)? preterm bebekler (< 32? gebelik haftas? veya? < 1500 g do?um a??rl???) ?al??maya al?nd?. Hidrokortizon, ilk hafta ba?lama dozu 1 mg/kg g?nde iki kez oral olarak, daha sonra klinik yan?ta g?re %10-20 azalt?larak uyguland?.
Bulgular: Toplam 90 infant ?al??maya al?nd?. Hidrokortizon tedavisinden sonra sadece ?? hasta solunumsal destek al?yordu. ?lac?n g?venilirli?i de?erlendirildi?inde 8 (%8.8) infantta hidrokortizon tedavisinin erken komplikasyonu g?zlendi.
Sonu?: ?al??mam?z, bildi?imiz kadar?yla literat?rde bronkopulmoner displazi tedavisinde hidrokortizon dozu ve ba?lama zaman? a??s?ndan ilk ?al??mad?r.
Anahtar Kelimeler: Bronkopulmoner displazi, hidrokortizon, preterm infant.
SUMMARY
Postnatal oral hydrocortisone in treatment of bronchopulmonary dysplasia: an observational study
Banu MUTLU, Gonca SANDAL, G?zde KANMAZ, Zeynep ERASLAN, ?mer ERDEVE, ?erife Suna O?UZ,
U?ur D?LMEN
Neonatal Intensive Care Unit, Ankara Dr. Zekai Tahir Burak Woman Health and Diseases Training and
Research Hospital, Ankara, Turkey.
Introduction: The aim of our study was? to determine whether hydrocortisone even at low dose could be an effective and safe alternative treatment for bronchopulmonary dysplasia.
Materials and Methods: This prospective pilot study was conducted in a tertiary referral neonatal intensive care unit placed in Ankara Zekai Tahir Burak Maternity Teaching Hospital.? Preterm babies (< 32 week gestational age or < 1500 g birth weight) who were ventilator dependent approximately at or beyond three weeks of age (defined as rescue treatment) or were oxygen dependent on postmenstrual 36th week without evidence of any infection (defined as bronchopulmonary dysplasia treatment) were enrolled in the study. Hydrocortisone was used orally in an initial dose of 1 mg/kg twice a daily for a week and then the dose was tapered by 10-20% every other day regarding to clinical response.
Results: A total of 90 infants were enrolled in this study. After hydrocortisone treatment only 3 (3.4%) patients were still on respiratory support. When safety of the drug was evaluated 8 (8.8%) infants had early complications of hydrocortisone treatment.
Conclusion: To the best of our knowledge this study is the first trial? in the literature along with the? hydrocortisone dose and the initiation time in treatment of bronchopulmonary displasia.
Key Words: Bronchopulmonary dysplasia, hydrocortisone, preterm infant.
Tuberk Toraks 2013; 61(3): 245-249 • doi:10.5578/tt.4066
Geli? Tarihi/Received: 06/08/2012 - Kabul Edili? Tarihi/Accepted: 14/06/2013
INTRODUCTION
Remarkable advances in the treatment of neonatal respiratory disorders, such as antenatal glucocorticoid therapy and alternative modes of ventilation, have reduced neonatal mortality and acute respiratory morbidity. However, bronchopulmonary dysplasia (BPD) remains as a substantial complication, especially among the most immature infants. Since inflammation plays a prominent role in pathogenesis of BPD, intravenous and oral steroids have been used as anti-inflammatory agents to alter the course of lung disease in premature infants (1,2,3).
Among all corticosteroids, dexamethasone has been well studied and has been found effective in weaning infants off mechanical ventilation when given moderately early (7-14 days of age) or late (14-28 days of age) in the initial nursery course (1,2,3). Despite this effect, dexamethasone therapy has not been shown to reduce the total days of hospitalization, duration of supplemental oxygen therapy or incidence of BPD (1). Unfortunately, dexamethasone therapy has been associated with an increase in cerebral palsy (CP) and other neurodevelopment impairments (NDI) at follow-up (1,4,5). Recent articles have suggested hydrocortisone as a safer alternative to dexamethasone in treating and preventing BPD, partly on the basis of data from observational long-term studies of fewer adverse behavioral, cardiovascular, neurological and immune outcomes, among others, in childhood (6,7,8,9,10). There has been a requirement to determine whether and on which doses hydrocortisone therapy is effective in treating BPD, and whether it is free of the adverse effects associated with systemic dexamethasone therapy.
This study was designed on the hypothesis of whether hydrocortisone even at low dose could be an effective and safe alternative treatment for BPD.
MaterialS and methods
This prospective study was conducted in a tertiary referral neonatal intensive care unit (NICU) placed in Ankara Dr. Zekai Tahir Burak Maternity Teaching Hospital.? The study was approved by the local Research Ethics Committee. Between January 2008 and August 2010 infants with BPD were recruited to this study. Infants were excluded if they had a diagnosis of suspected or proven infection, significant congenital malformation, and clinical evidence of PDA, NEC and intestinal hemorrhage or perforation. Parental informed concent was obtained for all enrolled patients.
Patient characteristics (gestational week, birth weight, gender, type of delivery etc.), maternal, prenatal and postnatal features, complications including infection, hyperglycemia, glycosuria, hypertension, necrotizing enterocolitis (NEC), gastrointestinal perforation, intraventricular hemorrhage (IVH), severe retinopathy of prematurity (ROP) were all recorded.
Preterm babies (< 32 week gestational age or < 1500 g birth weight) who were ventilator dependent approximately at or beyond three weeks of age (defined as rescue treatment) or were oxygen dependent on postmenstrual 36th week without evidence of any infection (defined as BPD treatment) were enrolled in the study. Hydrocortisone was used orally in an initial dose of 1 mg/kg twice a daily for a week and then the dose was tapered by 10-20% every other day regarding to clinical response. Clinical response was defined as reduced supplemental oxygen requirement (< 30%) and/or weaning from respiratory support (ventilation/n CPAP).
The major outcomes of the study were the efficacy and safety of the hydrocortisone treatment for BPD on 1 mg/kg dose beyond the third week of life. Patients who reached 12 months were evaluated for neurodevelopment. Development was assessed with the Bayley Scales of Infant Development II. Mental Development Index (MDI) or Psychomotor Development Index (PDI) > 2 SD below the mean (ie, < 70) was defined as abnormal. All Bayley II examiners were previously certified.
RESULTS
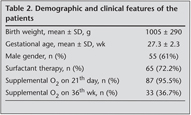
A total of 90 infants were enrolled in this study. Of these, 3 died during the hospitalization and left 87 were available for follow-up. Among these infants, 37 (42.5%) who reached 12 months adjusted age were evaluated for long-term neurodevelopmental outcome.
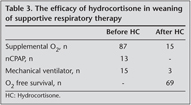
The demographic and clinical features of the patients? are summarized in Table 1. Maternal demographic charecteristics are summarized in Table 2. Thirty two (35.5%) infants who needed respiratory support as mechanical ventilation or nasal CPAP received rescue hydrocortisone treatment after 3rd week of life whereas 58 (64.4%) infants who were beyond 36th postmenstrual week and on oxygen and/or pressure support received treatment for BPD.
Mean duration of hospitalization was 79.5 ? 24.4 days and mean initiation time of hydrocortisone was 41.8 ? 20.1 days. The effect of hydrocortisone treatment on respiratory support is shown in Table 3. Mean duration of hydrocortisone treatment was 22.5 ? 14 days. After treatment only 3 (3.4%) patients were still on respiratory support. Three patients who did not respond to hydrocortisone received dexamethasone for extubation. Overall, 15 (16.6%) infants were discharged with home oxygen despite the hydrocortisone or dexamethasone treatment (Table 3).
When safety of the drug was evaluated 8 (8.8%) infants had early complications of hydrocortisone treatment such as hypertension (2), cardiomyopathy (3) and sepsis (3).? Neurodevelopmental outcomes are summarized in Table 4.
DISCUSSION
The aims of this study were to investigate whether hydrocortisone was safe and efficacious for extubation of very low birth weight infants who still needed pressure support beyond third week of life, and whether it altered progress of BPD. This study shows that hydrocortisone is a potent in reducing supplemental oxygen and ventilator dependency. It further provides evidence that acute side effects, related to neonatal glucocorticoid therapy are scarce and acceptable.
The use of oral and intravenous steroids has been studied extensively in infants with BPD. There is a little doubt that when administered early and moderately early, they facilitate weaning from mechanical ventilation and extubation. Since the use of dexamethasone in preterm very low birth weight infants to prevent BPD has not been recommended any longer because of the obvious increases in neurologic complications, there is an increased tendency towards to use different types of steroids which is thought to be safer such as hydrocortisone (11).
Doyle et al. reported a systematic review and meta-analysis of the RCTs of hydrocortisone in preterm neonates and identified a total of eight trials (11). In all of these studies hydrocortisone was started in the first week of life before BPD was established. In all except one study the dose of hydrocortisone was as 1 mg/kg and they concluded that there was no evidence to support the use of hydrocortisone to prevent BPD. On contrary, we demonstrated that hydrocortisone at 1 mg/kg dose was effective to wean patients from respiratory support in patients who progress to BPD or had BPD. Reports of hydrocortisone therapy given to facilitate extubation have been limited to cohort studies. In the first reported study, 25 infants treated with hydrocortisone at one hospital (5 mg/kg per day, tapered over three weeks) were compared with 25 untreated infants at the same hospital and additionally with a cohort of 23 infants treated with dexamethasone (0.5 mg/kg per day, tapered over three weeks) at a separate hospital (12). The investigators found that hydrocortisone was an effective as dexamethasone in weaning infants from the ventilator and in decreasing supplemental oxygen therapy, with fewer short-term adverse effects. Follow-up of these children at school age revealed no differences in neurodevelopmental outcomes between their comparison groups whereas dexamethasone treated infants more often had an abnormal neurologic examination and less favorable school performance than their comparison cohort. Although we also demonstrated high success rate of hydrocortisone in facilitating extubation, being observational study neurodevelopment results of our patients could only give idea about our cohort. Compared with preterm infants not treated with hydrocortisone, no differences have been found in neurocognitive or motor outcome, or in the incidence of brain abnormalities on magnetic resonance imaging, after hydrocortisone treatment for bronchopulmonary dysplasia in long-term follow-up studies at 5-8 years of age (6,7,13,14,15), besides hydrocortisone treated children were younger, smaller and sicker than their untreated comparison group. Importantly these children were treated exclusively with hydrocortisone and there was no contamination by later prescription of dexamethasone. However, the patient population was retrospectively enrolled and hydrocortisone administration did not commence until after the first postnatal week. Waterberg et al. and Peltoniemi et al. used early hydrocortisone treatment and both reported no adverse effects on neurodevelopmental outcomes at 18 to 22 months' corrected age (16,17).
Potential complications of prolonged corticosteroid therapy include infection, hypertension, hyperglycemia, adrenocortical suppression, somatic and lung growth suppression, hypertrophic cardiomyopathy and gastrointestinal catastrophes' such as bleeding, perforation and necrotizing enterocolitis. In this study we observed some side effects including, hypertension (n= 2), hypertrophic cardiomyopathy (n= 3) and infection (n= 3) but the other mentioned side effects did not occur in the patient cohort. In two early treatment (prophylactic) hydrocortisone studies, enrolment was stopped because of an increased incidence of spontaneous gastrointestinal perforation with combined hydrocortisone-indomethacin treatment (18,19). In very low birth weight infants, low-dose hydrocortisone alone (in the absence of co-treatment with prophylactic indomethacin) did not lead to gastrointestinal perforation. More studies are needed to examine the type and safe dose of corticosteroid in weaning patients from respiratory support and treatment of BPD. To our knowledge this study is unique in the literature along with the hydrocortisone dose and the initiation time. Since present study was an observational one, our results should provide some guidance for the late use of hydrocortisone in infants with BPD. There is a need for prospective randomized controlled trials which will verify the effectiveness and safety of this practice.
CONFLICT of INTEREST
None declared.
REFERENCES
- Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2003: CD001146
- Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2003: CD001144
- Halliday HL, Ehrenkranz RA, Doyle LW. Delayed (> 3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev 2003: CD001145.
- Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants < 32 weeks' gestation between 1993 and 1998. Pediatrics 2005; 116: 635-43.
- Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Eng J Med 2004; 350: 1304-13.
- Rademaker KJ, de Vries LS, Uiterwaal CS, Groendaal F, Grobbee DE, van Bel F. Postnatal hydrocortisone treatment for chronic lung disease in the preterm newborn and long-term neurodevelopmental follow-up. Arch Dis Child Fetal Neonatal Ed 2008; 93: F58-F63.
- Karemaker R, Heijnen CJ, Veen S, Baerts W, Samson J, Visser GH, et al. Differences in behavioural outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res 2006; 60: 745-50.
- Karemaker R, Karemaker JM, Kavellaars A, Tersteeg-Kamperman M, Baerts W, Veen S, et al. Effects of neonatal dexamethasone treatment on the cardiovascular stress response of children at school age. Pediatrics 2008; 122: 978-87.
- Karemaker R, Kavelaars A, ter Wolbeek? M, Tersteeg-Kamperman M, Bearts W, Veen S, et al. Neonatal dexamethasone treatment for chronic lung disease alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics 2008; 121: e870-e878.
- Rademaker KJ, de vries WB. Long term effect of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Semin Fetal Neonatal Med 2009; 14: 171-7.
- Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010; 98: 111-7.
- Hassan AH, von Rosenstiel P, Patchev VK, Holsboer F, Almedia OF. Exacerbation of apoptosis in the dentate gyrus of the aged rat bu dexamethasone and the protective role of corticosterone. Exp Neurol 1996; 140: 43-52.
- van der Heide-Jalving M, Kamphuis PJ, van der Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, et al. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatr 2003; 92: 827-35.
- Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, et al. Structural and functional brain development after hydrocaortisone treatment for neonatal chronic lung disease. Pediatrics 2005; 116: 1-7.
- Rademaker KJ, Rijpert M, Uiterwaal CS, Lieftink AF, van Bel F, Grobbee DE, et al. Neonatal hydrocortisone treatment related to 1H-MRS of the hippocampus and short-term memory at school age in preterm born children. Pediatr Res 2006; 59: 309-13.
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007; 120: 40-8.
- Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, et al; Neonatal Hydrocortisone Working Group. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology 2009; 95: 240-7. doi: 10.1159/000164150.
- Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 2004; 114: 1649-57.
- Peltoniemi O, Kari MA, Heinonen K, Saarela T, Nikolajev K, Andersson S, et al. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high-risk infants. J Pediatr 2005; 146: 632-7.
Yaz??ma Adresi (Address for Correspondence):
Dr. Gonca SANDAL,
Ankara
Dr. Zekai Tahir Burak Kad?n Sa?l??? E?itim ve
Ara?t?rma Hastanesi,
Yenido?an Yo?un Bak?m ?nitesi, ANKARA - TURKEY
e-mail: kocabasgonca@mynet.com