Obstr?ktif uyku apne sendromu tan?l? hastalarda bron? hiperreaktivitesi
Emel BULCUN, Mehmet EK?C?, Aydanur EK?C?, G?khan T?REL?, T?lay KARAKO?, Erol ?ENT?RK,
Volkan ALTINKAYA
K?r?kkale ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, K?r?kkale.
?ZET
Obstr?ktif uyku apne sendromu tan?l? hastalarda bron? hiperreaktivitesi
Giri?: Obstr?ktif uyku apne sendromu (OUAS) ve bron? hiperreaktivitesi (BHR) aras?ndaki ili?ki iyi bilinmemektedir. Bu ?al??mada, OUAS'l? hastalarda hastal???n ?iddeti ve BHR aras?ndaki ili?kiyi ara?t?rd?k.
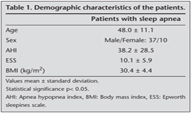
Materyal ve Metod: Bu ?al??maya polisomnografileriyle OUAS tan?s? alan 47 (37'si erkek, 10'u kad?n) hasta al?nd?. Histamin provokasyon testi yap?ld? ve beden kitle indeksi (BK?, kg/m2) hesapland?. BHR'nin varl??? bron? provokasyon test (BPT) pozitifli?i (PD de?erleri ≤ 16 mg/mL) olarak tan?mland?. Hastalar Epworth uyku skalas? (EUS) ile sorguland?.
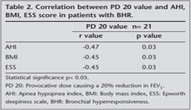
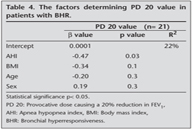
Bulgular: K?rk yedi hastan?n 21'inde histamin provokasyon testi pozitifti. BHR'li hastalarda PD 20 de?eri ile apne-hipopne indeksi (AH?) (r= -0.47, p= 0.03), BK? (r= -0.45, p= 0.03) ve EUS skoru (r= -0.45, p= 0.03) aras?nda negatif ili?ki vard?. Bununla birlikte, BHR'li hastalarda (21 hasta) AH? (p= 0.03), BK? (p= 0.02), EUS skoru (p= 0.03) BHR'ye sahip olmayan hastalardan (26 hasta) daha y?ksekti. Multipl regresyon analizinde ya? ve cinsiyetten ba??ms?z olarak PD de?eri ve AH? (b= -0.45, p= 0.03) aras?nda negatif ili?ki bulundu ve BHR'nin varl??? ile AH? (p= 0.04) ve BK? (p= 0.03) aras?nda pozitif ili?ki bulundu.
Sonu?: OUAS'l? hastalarda BHR yayg?nd?r. OUAS'?n ?iddeti artt?k?a BHR'nin ?iddeti artar. Bununla birlikte, obezite OUAS'l? hastalarda BHR'nin varl???n? tetikleyebilir.
Anahtar Kelimeler: Obstr?ktif uyku apne sendromu, bron? hiperreaktivitesi, obezite.
SUMMARY
Bronchial hyperresponsiveness in patients with obstructive sleep apnea syndrome
Emel BULCUN, Mehmet EK?C?, Aydanur EK?C?, G?khan T?REL?, T?lay KARAKO?, Erol ?ENT?RK,
Volkan ALTINKAYA
Department of Chest Diseases, Faculty of Medicine, Kirikkale University, Kirikkale, Turkey.
Introduction: The relationship between obstructive sleep apnea syndrome (OSAS) and bronchial hyperresponsiveness (BHR) is not well known. In this study, we investigated the association between BHR and disease severity in patients with OSAS.
Materials and Methods: Fourty seven (37 male/10 female) OSAS patients admitted with polysomnography enrolled to the study. Histamine bronchial challenge test was performed and body mass index (BMI, kg/m2) was calculated. Presence of BHR was diagnosed as positivity of bronchial provocative test (BPT) (PD values ≤ 16 mg/mL). Patients were questioned with Epworth sleepiness scale (ESS).
Results: Histamine bronchial challenge test was positive in 21 of 47 patients. There were significant negative correlations between PD 20 value and AHI (r= -0.47, p= 0.03), BMI (r= -0.45, p= 0.03), and ESS score (r= -0.45, p= 0.03) in the patients with BHR. In addition, AHI (p= 0.03), BMI (p= 0.02), ESS scores (p= 0.03) were higher in patients with BHR (21 patients) than in patients not having BHR (26 patients). Significant negative relation was found between PD 20 value and AHI (b=-0.45, p= 0.03) and significant positive relation was found between presence of BHR and AHI (p= 0.04), BMI (p= 0.03) independently of age and sex in multiple regression analysis.
Conclusion: BHR is common in patients with OSAS. As severity of OSAS increased, severity of BHR increased. In addition, obesity may trigger presence of BHR in patients with OSAS.
Key Words: Obstructive sleep apnea syndrome, bronchial hyperresponsiveness, obesity.
Tuberk Toraks 2013; 61(3): 221-226 • doi:10.5578/tt.5791
Geli? Tarihi/Received: : 02/02/2013 - Kabul Edili? Tarihi/Accepted: 08/07/2013
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is characterised by repetitive episodes of upper airway occlusion during sleep. OSAS has been shown to be associated with a variable degree of nasal inflammation, uvula mucosal congestion and bronchial hyperresponsiveness (BHR) (1). In various studies, the presence of neutrophilic inflammation has been detected in lower airways in induced sputum (2,3). It has been reported that sleep apnea in patients diagnosed with asthma may trigger asthma episodes and nasal CPAP which is the conventional treatment for OSAS may be safely used in these patients and this treatment may control asthma episodes, especially nocturnal symptoms (4). Various studies established the presence of BHR in OSAS patients without asthma. However, conflicting results have been obtained on BHR in patients with OSAS according to these studies. In the study of Devouassoux et al, the rate of BHR has been found to be 11% in OSAS patients (2). Nevertheless, BHR was found at the rate of 22% and 25% in in the studies of K?kt?rk and Lin respectively and both studies reported no correlation between the severity of BHR and severity of disease (5,6).
The aim of this study was to investigate the presence of BHR in patients with OSAS. In addition, in this study, we examined the factors determining severity of BHR in this patients.
MAterials and Methods
Study Design
Fourty seven patients with the complaints of snoring, witnessed apnea and daytime sleepiness who were diagnosed with OSAS based upon polysomnography results. Informed consent was taken from all patients in the beginning of the study and the study was approved by the local ethical committee. Asthma, bronchiectasis, chronic obstructive pulmonary disease, active smokers, severe systemic diseases, allergic rhinitis, pregnancy, were accepted as exclusion criteria. Patients who had been asthma symptoms such as episodic breathlessness, wheezing, cough, chest tightness were not included. Body mass index (BMI) (kg/m2) was calculated by measuring weight and height. Pulmonary function tests (PFT) were performed with flow sensitive spirometer. Histamine bronchial challenge tests were performed. Their sleeping status was inquired with Epworth sleepiness scale (ESS).
Pulmonary Function Tests
PFT were performed with flow sensitive spirometry (Sensor Medics?, Vmax spectra 22, USA) according to "American Thoracic Society" (ATS) criterias (7). Forced vital capacity % (FVC%), forced expiratory volume in one second % (FEV1), peak expiratory flow % (PEF%) and FEV1/FVC ratio were recorded.
Epworth Sleepiness Scale (ESS)
The ESS is simple, eight item self-administered scale wich is widely used in clinical practice to quantify the level of daytime sleepiness in situations of different soporificity. Is has a total score range of 0-24 and scores > 10 are indicative of excessive daytime sleepiness (8).
Bronchial Provocation Test
Patients were assessed with a histamine inhalation test to determine their level of BHR. Histamine solution (diphosphate salt, Sigma, Diesenhofen, Germany) was prepared in sterile isotonic saline. The histamine bronchial challenge test was performed according to a standardized procedure (9). Pulmonary functions were measured with a flow-sensing spirometer connected to a computer. LAB, version 4.3 software (Jeager, Wurzburg, Germany) was used for the analysis. Each subject inhaled increasing concentrations of histamine (0.03-16 mg/mL), nebulized by a dosimeter with a mean output of 9 ?L/puff (SD_0.3) (Dosimeter APS Pro, Jeager, Wurzburg, Germany), until FEV1 was reduced by 20% from baseline values. Bronchial response to histamine was expressed as the provocative dose (mg/mL) causing a 20% reduction in FEV1 (PD 20) and was calculated by using LAB, version 4.3 software (Jeager, Wurzburg, Germany). A concentration of histamine < 16 mg/mL was taken as positive bronchial provocative test (BPT) result. Presence of BHR was diagnosed as positivity of BPT (PD values ≤ 16 mg/mL).
Sleep Study
Overnight polysomnography was performed in all patients by a computerized system (Sensormedics, USA) and included the following variables: electrooculogram (two channels), electroencephalogram (four channels), electromyogram of submental muscles (two channels), electromyogram of the anterior tibialis muscle of both legs (two channels), and electrocardiogram and airflow (oro-nasal canules). Chest and abdominal efforts (two channels) were recorded using inductive plethysmography, arterial oxyhemoglobin saturation (SaO2: 1 channel) by pulse oximetry with a finger probe. Sleep was scored using the criteria of Rechtschaffen and Kales for epochs of 20 s by a scorer experienced in the use of standard guidelines (10). Sleep stages were scored using standard criteria. Apnea was defined as cessation of airflow for 10 s. Hypopnea was defined as a 30% reduction of airflow or respiratory movements accompanied by a 3% decrease in SaO2 and/or followed by an arousal. The AHI was established as the ratio of the number of apnoeas and hypopnoeas per hour of sleep. Subjects with an AHI of < 5 were classified as nonapneic snorers (11).
Statistical Analysis
Results are expressed as mean ? standard deviation (SD). Significance of difference between groups was assessed by unpaired student's t-test. The relation between presence of BHR and AHI, ESS score and BMI was investigated with "pearson correlation test". In "multiple linear regression"analysis, the relation of PD 20 value with AHI, BMI, age and sex, was investigated in patients with BHR. In additon, in "logistic regression"analysis, the relation of presence of BHR with AHI, BMI, age and sex was investigated in all patients with sleep apnea. The statistical analysis was performed using the SPSS program (SPSS Inc., IL, USA) and p-values were two tailed analysed. p values of less than 0.05 were considered statistically significant.
RESULTS
The study included 37 male and 10 female patients. The mean age was 48.0 ? 11.1 (minimum: 21-maximum: 76) years, the mean BMI was 30.4 ? 4.4 kg/m2, the mean AHI was 38.2 ? 28.5 events per h, the mean ESS scores was 10.1 ? 5.9 (Table 1). BHR was showed in 21 (16 male/5 female) of 47 (44%) patients. There were significant negative correlations between PD 20 values and AHI (r= -0.47, p= 0.03), BMI (r= -0.45, p= 0.03), and ESS score (r= -0.45, p= 0.03) in the patients with BHR according to pearson correlation analyses (Table 2). AHI (p= 0.03), BMI (p= 0.02), ESS scores (p= 0.03) were higher in patients with BHR (21 patients) than in patients without BHR (26 patients) (Table 3). In multivariable linear regression analysis, the relation between PD 20 value and age, sex, AHI and BMI was analysed in the patients with BHR. Statistically significant negative relation was found between PD 20 values and AHI (b = -0.45, p= 0.03). However, PD 20 values did not show significant association with BMI, age, sex (Table 4). Besides, in logistic regression analysis, the relation between presence of BHR and age, sex, AHI and BMI was analysed in the all of patients with sleep apnea. Statistically significant positive relative was found between presence of BHR and AHI (p= 0.04) and BMI (p= 0.03) but presence of BHR did not show significant association with age, sex (Table 5).
DISCUSSION
In the present study, rate of BHR in patients with OSAS was as high as 44%. The presence and severity of BHR were correlated with severity of sleep apnea (AHI), BMI and ESS score according to pearson correlation analyses. In addition, AHI was determining presence and severity of BHR and BMI were determining only presence of BHR in multivariate analysis. As severity of OSAS increased, presence and severity of BHR increased. In additon, this results showed that BMI was also increased presence of BHR in patients with sleep apnea.
In the literature, in a large cohort of OSAS patients, airway hyperresponsiveness to cold air has been reported as 4% (12). In addition, previous studies on BHR in patients with OSAS have indicated equivocal results. When Lin et al. used maximum metacholin dose at 25 mg, they established 25% BHR in patients with OSAS (5). The other study showed that three patients had BHR on methacholine challenge among sexteen patients with OSAS (13). In the our study, maxium histamin dose was 16 mg and BHR was established at the rate of 44%. In other studies, there was not the relation between severity of OSAS and BHR (4,5). However, Livy et al. established that at 2nd-3rd month of CPAP treatment in OSAS patients BHR was decreased (5). Devoassoux et al. used 4 mg as maximum metacholin dose and found 11% BHR in patients with OSAS (2). They found BHR as 40% at the first weeek of CPAP treatment and as 33% at fourth week in the patients with OSAS, they explained that BHR increased with CPAP treatment. Various mechanisms have been proposed to explain BHR in patients with OSAS. Hypoxia can trigger BHR by the mechanisms through stimulation of the carotid bodies resulting in reflex bronchoconstriction. There are repeated stimulation of glossopharynx, glottis and larynx in snoring patients. Neural receptors in the glottis and in the laryngeal region have reflex bronchoconstrictive activity. Muller maneuvers, which is a potent vagus stimulator are observed in OSAS patients frequently and vagal hyperfunction causes bronchial hyperresponseveness (14,15,16,17). In patients with OSAS, increases in bronchial inflammation was defined as increase neutrophils, IL-8 counts in induced sputum, and increase NO in exhaled air (4,5). The repeated mechanical trauma on the airway vibration and the forceful suction collapse during apneas, likely triggers an inflamatory response locally (18). Bronchial inflammation is thought to facilitate BHR development (19).
Although several mechanisms underlying the BHR-obesity connection have been proposed, debates still remain. The effect of obesity on BHR have been examined in a lot of studies. It was suggested that subcutaneous abdominal fat was significantly associated with BHR (20). Litonjua et al. reported that high initial BMI was associated with an increased risk of developing BHR (21). In the other study was compared BHR between normal and increased weight women and this study suggested overweight or obese women showed a higher prevalence of symptomatic BHR (22). Obesity is very common in patients with OSAS. The results of sleep apnea and obesity are similar to each other, such as increased cardiovascular disease and increased mortality rates. While obesity is the primary risk factor for the development systemic inflammation and sleep apnea, sleep apnea may increases the inflammatory and metabolic disorders (23,24). Obesity as a state of chronic systemic inflammation resulting from interactions between adipocytes and adipose tissue macrophages that are recruited to obese adipose tissue. This inflammation, particularly obesity-related changes in TNF-a, leptin, and adiponectin, may contribute to airway hyperresponsiveness in obesity (25). In the present study, both severity of sleep apnea and BMI were determining presence of BHR as independent of each other. In this study we showed that the effect of obesity on severity of BHR did not reach statistical significant according to multiple linear regression analysis because our study had a small number of patients. In support of this b value of BMI was higher enough in multivariable linear regression analysis examining the relation between degree of BHR and BMI. Besides, it was showed the correlation between degree of BHR (PD values) and BMI in the simple pearson analyses.
In conclusion, BHR is common in patients with OSAS. Degree of BHR increases depending on severity of sleep apnea and accompanying obesity. In addition, patients with BHR is more obese, their disease is more severe. Presence of BHR in OSAS should be taken into account.
CONFLICT of INTEREST
None declared.
REFERENCES
- Sabato R, Guido P, Salerno FG, Resta O, Spanevello A, Barbaro MP. Airway inflammation in patients affected by obstructive sleep apnea. Monaldi Arch Chest Dis 2006; 65: 102-5.
- Devouassoux G, L?vy P, Rossini E, Pin I, Fior-Gozlan M, Henry M, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 2007; 119: 597-603.
- Salerno FG, Carpagnano E, Guido P, Bonsignore MR, Roberti A, Aliani M, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 2004; 98: 25-8.
- K?kt?rk O, F?rat H. Bronchial hyperreactivity in patients with obstructive sleep apnea syndrome. Diagnosis and treatment of sleep breathing disorders. Alpes Congres, Grenoble, Fransa 1998; 67 (P-69).
- Lin CC, Lin CY. Obstructive sleep apnea syndrome and bronchial hyperreactivity. Lung 1995; 173: 117-26.
- Kokturk O, Ciftci B. Overlap syndrome. Tuberk Toraks 2003; 51: 333-48.
- American Thoracic Society. Standardization of spirometry. 1987 update. Am Rev Respir Dis 1987; 136: 1285-98.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale Sleep 1991; 14: 540-5.
- Foresi A, Mattoli S, Corbo GM, Polidori G, Ciappi G. Comparison of bronchial responses to ultrasonically nebulized distilled water, exercise, and methacholine in asthma. Chest 1986; 90: 822-6.
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages in human subjects, Brain Information Service, VCLA, Los Angeles, CA, 1968.
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 2nd ed. Diagnostic and Coding Manual, American Academy of Sleep Medicine, Westchester, IL 2005.
- Thalhofer S, Dorow P, Meissner P, Luding K. Change in bronchial hyperreactivity with nCPAP respiration in patients with sleep related respiratory disorders. Pneumologie 1997; 51(Suppl 3): 767-9.
- Sar?man N, Levent E, Cubuk R, Yurtlu S, Benli Aksungar F. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath 2011; 15: 341-50. doi: 10.1007/s11325-010-0387-7.
- Denjean A, Canet E, Praud JP, Gaultier C, Bureau M. Hypoxia-induced bronchial responsiveness in awake sheep: role of carotid chemoreceptors. Respir Physiol 1991; 83: 201-10.
- Guilleminault C, Quera-Salva MA, Powell N, Riley R, Romaker A, Partinen M, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J 1988; 1: 902-7.
- Nadel JA, Widdicombe JG. Reflex Effects upper airway irritation on total lung resistance and blood pressure. Jappl Physiol 1962; 17: 861-5.
- Martin RJ. Characteristics and mechanisms of nocturnal asthma. Allergy Proc 1993; 14: 1-4.
- Paulsen FP, Steven P, Tsokos M, Jungmann K, M?ller A, Verse T, et al. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med 2002; 166: 501-9.
- Huang TJ, Haddad EB, Fox AJ, Salmon M, Jones C, Burgess G, et al. Contribution of bradykinin B(1) and B(2) receptors in allergen-induced bronchial hyperresponsiveness. Am J Respir Crit Care Med 1999; 160: 1717-23.
- Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, et al. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc 2011; 32: 68-73. doi: 10.2500/aap.2011.32.3407.
- Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax 2002; 57: 581-5.
- Sood A, Dawson BK, Eid W, Eagleton LE, Henkle JQ, Hopkins-Price P. Obesity is associated with bronchial hyper-responsiveness in women. J Asthma 2005; 42: 847-52.
- Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: pathophysiology, complications, and treatment. Nutr Clin Pract 2009; 24: 675-87.
- L?vy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 2009; 34: 243-60.
- Stephanie A. Shore. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol 2010; 108: 735-43.
Yaz??ma Adresi (Address for Correspondence):
Dr. Emel BULCUN,
K?r?kkale ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
KIRIKKALE - TURKEY
e-mail: emelbulcun@hotmail.com