"Asthma
quality of life questionnaire" ya?am kalitesi anketinin
eri?kin ast?ml? T?rk
hasta ?rne?inde ge?erlili?i ve g?venilirli?i
Aylin
?ZGEN ALPAYDIN1, Arzu YORGANCIO?LU1, ?zge YILMAZ2,
Mine BORA1, Tu?ba G?KTALAY1,
P?nar ?EL?K1,
Hasan Y?KSEL2
1 Celal Bayar ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Manisa,
2 Celal Bayar ?niversitesi T?p Fak?ltesi, ?ocuk Sa?l??? ve Hastal?klar? Anabilim Dal?, Pediatrik Allerji Bilim Dal? ve
Solunum Birimi, Manisa.
?ZET
"Asthma quality of life questionnaire" ya?am kalitesi anketinin eri?kin ast?ml? T?rk hasta ?rne?inde ge?erlili?i ve g?venilirli?i
?al??mam?zda? Ast?m ya?am kalitesi anketi [Asthma Quality of Life Questionnaire (AQLQ)]'nin T?rk?e s?r?m?n?n eri?kin ast?ml? T?rk hastalarda ge?erlili?i ve g?venilirli?inin ara?t?r?lmas? ama?lanm??t?r. Ast?m i?in k?resel giri?im [Global Initiative for Asthma (GINA)] 2008 kriterlerine g?re daha ?nceden veya yeni tan? alm?? 18-55 ya? aras? stabil d?nemde, semptomatik, ard???k 118 ast?m hastas? ?al??maya al?nd?. Hastalar?n ast?m a??rl??? belirlendi ve AQLQ anketinin T?rk?e adaptasyonu uyguland?. Ayn? zamanda Lara ast?m semptom skorlar? (LASS), solunum fonksiyon testleri, "Medical Outcomes Survey Short Form-36 (SF-36)" T?rk?e adaptasyonu de?erlendirildi. T?m uygulamalar ba?lang??ta ve 10. haftada yap?ld?. Bu s?re? i?inde hastalar tedavilerini gere?inde de?i?tirmekte serbest b?rak?ld?lar. ?al??maya al?nan 118 hastan?n 95'i kad?nd?, 14 hasta takipte de?erlendirilemedi. Hastalar?n %62'si hafif, %38'si orta-a??r ast?m grubundayd?. AQLQ i? uyumlulu?u y?ksekti (Cronbach's alpha 0.81-0.87) ve soru-toplam skor korelasyonlar? 0.75-0.89 aras?nda de?i?mekteydi. AQLQ alan ve toplam skorlar? ile SF-36 alan skorlar? aras?ndaki kesitsel ve izlemsel ili?ki az veya orta derece aras?nda de?i?mekteydi (r= 0.241-0.626, p< 0.005). AQLQ de?erleri ast?m a??rl??? ve LASS'ye g?re birinci (p< 0.001, her ikisi i?in) ve 10. hafta vizitlerinde (p= 0.006, p< 0.001 s?ras?yla) anlaml? derecede farkl?l?k g?stermekteydi. ?zlemde LASS'nin anlaml? de?i?iklik g?stermesine paralel olarak, AQLQ'nun semptom domaininde anlaml? de?i?iklik oldu?u saptand? (p< 0.001). Sonu?lar?m?z AQLQ'nun T?rk?e s?r?m?n?n eri?kin ast?ml? T?rk hastalarda uygulanabilir, ge?erli ve g?venilir oldu?unu ortaya koymu?tur.
Anahtar Kelimeler: Ast?m, ya?am kalitesi, ge?erlilik, g?venilirlik.
SUMMARY
Validity and reliability of "asthma quality of life questionnaire" in a sample of Turkish adult asthmatic patients
Aylin
?ZGEN ALPAYDIN1, Arzu YORGANCIO?LU1, ?zge YILMAZ2,
Mine BORA1, Tu?ba G?KTALAY1,
P?nar ?EL?K1,
Hasan Y?KSEL2
1 Department of Chest Diseases, Faculty of Medicine, Celal Bayar University, Manisa, Turkey,
2 Division of Pediatric Allergy and Respiration Unit, Department of Children's Health and Diseases,
Celal Bayar University, Manisa, Turkey.
We aimed to investigate the validity and reliability and of "Asthma Quality of Life Questionnaire (AQLQ)" in Turkish adult asthmatic patients. New or previously diagnosed [according to Global Initative for Asthma (GINA) 2008] symptomatic 118 consecutive stable asthmatic patients between 18 and 55 years old were included. Asthma severity was determined and Turkish adaptation of the AQLQ was administered. Lara asthma symptom scales (LASS), pulmonary function tests, Turkish adaptation of Medical Outcomes Survey Short Form-36 (SF-36) were evaluated. All assessments were done twice at recruitment and after 10 weeks. During this period patients were allowed to make modifications on their medication when necessary. Among the recruited 118 patients 95 were female and 14 were lost in the follow-up. Sixty-two percentages of the patients had mild and 38% moderate asthma. The internal consistency of AQLQ was high (Cronbach's alpha 0.81-0.87) and item-total score correlations were ranging from 0.75-0.89. The cross-sectional and longitudinal correlations between AQLQ total and domain scores and SF36 domain scores were in a range of little or fair degree (r= 0.241-0.626, p< 0.005). Total AQLQ scores were observed significantly different according to disease severity and LASS both in the first (p< 0.001, both) and 10 weeks follow-up visits (p= 0.006, p< 0.001 respectively). A statistical significant change was observed in AQLQ symptom score as in total LASS changed (p< 0.001, both) in the follow-up. Our results demonstrated that Turkish version of AQLQ is feasible, reliable, valid and sensitive to changes in adult asthmatics.
Key Words: Asthma, quality of life, validity, reliability.
Quality of life is a concept associated with different aspects of human life. Health-Related Quality of Life (HRQOL) focuses on individuals' health status and impacts of the administered treatments. Measuring HRQOL in clinical investigations for assessing disease severity as well as evaluating the efficiency of the therapies in patients with chronic lung disease has become a popular trend in the recent years (1,2). Clinical evaluation determines a patient's health status in terms of objective methods, however a favorable assessment of health status should also include patient's own perception of his condition (3). Asthma is a chronic respiratory disease with significant impacts on physical, emotional, and social life (4). Therefore, besides physiological and clinical evaluations, assessment of health related impairment of quality of life has been an important issue as an outcome measure of asthma (5).
Most commonly used quality of life instruments specific for asthma in adults are the "Asthma Quality of Life Questionnaire (AQLQ)" "Living with Asthma Questionnaire" and "Chronic Obstructive Pulmonary Disease and Asthma St. George's Respiratory Questionnaire" (6,7,8). The AQLQ was developed by Juniper et al. and designed to be used in clinical trials (6). It has been shown to be reliable (ability to measure differences between patients) and valid (correlation with other indices of quality of life) in asthmatic patients (9). Usually, quality of life questionnaires are generated in English and translated to other languages (10). Reliability and validity of HRQOL questionnaires should be evaluated in each country before using these instruments in multicentric international studies although they are proven to be so in their original forms (11). Therefore we conducted a study in our community to determine the reliability, cross-sectional and longitudinal validity, internal consistency and responsiveness of the Turkish version of the AQLQ in the assessment of disease's impact on physical and, mental state of the asthmatic patients.
MATERIALS and METHODS
Asthma Quality of Life Questionnaire
Juniper AQLQ is a disease specific quality of life questionnaire including 32 items, in four health domains as activity limitation (11 items) (item nos. 1, 2, 3, 4, 5, 11, 19, 25, 28, 31, 32), symptoms (12 items) (item nos. 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 29, 30), emotional function (5 items) (item nos. 7, 13, 15, 21, 27) and environmental stimuli (4 items) (item nos. 9, 17, 23, 26). This instrument measures quality of life over the two weeks prior to the interview.
Scoring of the AQLQ
Each item within the AQLQ is equally weighted. The patients select from an ordinal scale of 7 point,-Likert type answer for every item. The domain scores are computed as the means of domain-specific items and global AQLQ score is computed as the mean of the domain scores. The score of each domain and the global score range from 1 to 7, corresponding to no impairment and maximum impairment, respectively, in quality of life. The minimum clinically important difference for AQLQ has been reported as 0.52 (12).
Turkish Adaptation Process
The original English version of the AQLQ has already been adapted to Turkish by four independent Turkish bilingual senior translators (CS, SE, EE, HF). Translation has been made in colloboration with MAPI Research Institute. In this study, this Turkish version of AQLQ has been used.
Patients
Between January and July 2010, a total number of 118 asthmatic patients who admitted to our respiratory outpatient department were included in the study consecutively They had clinically documented asthma and reversible airflow limitation was demonstrated with an improvement equal to or greater than 15% and 200 mL of forced expiratory volume in 1 second (FEV1) after inhalation of 200 ?g of salbutamol or with a positive methacholine test. Patients in the stable state with medication and aged between 18 and 55 years were included. Patients with other forms of airway obstruction or known acute or chronic comorbidities interfering with physical and psychological performance were excluded.
Study Design
Asthma symptom scores defined by Lara et al. were asked (13). The AQLQ, pulmonary function tests and Medical Outcomes Survey Short Form 36 (SF-36) were performed, thus a cross sectional evaluation was possible (13). After a 10 weeks period patients were asked to complete the AQLQ, SF-36 and asthma symptom scores again and pulmonary function tests were repeated. During this period, patients were allowed to make alterations on their medication when necessary.
All questionnaire data were collected by authors during face-to-face interviews. The method of filling out the questionnaire was explained and patients provided written informed consent. The study was approved by the human-research review board.
Measurement Scales
Lara asthma symptom scale (LASS) include 8 items including cough, wheezing, shortness of breath, chest pain, night symptoms, perceived asthma severity and attacks in the last four weeks. These items were categorized into a Likert-like 5-point scale as (never, a few days, some days, most days, everyday) with the exception of total number of attacks. Attack numbers are recorded and further categorized as; 1= no attacks in the last month, 5 ≥ 3 attacks a month, thus attacks are evaluated by two items. The LASS has been reported to have good internal consistency, excellent validity based in a heterogeneous sample of adults with persistent asthma (14).
SF-36 is a generic HRQOL questionnaire whose validation has been done in Turkish population (15). It is composed from 36 items in 8 domains as physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health. The score from the subscales for this questionnaire range from 0 to 100, with 0 representing no deterioration in the quality of life. Total score of the questionnaire is not calculated; however two summary scores for physical and mental components can be determined from the domain scores. In general population the component summary scores are standardized to have a mean value of 50 with a standard deviation of 10.
Pulmonary function tests were performed with Jaeger Master Screen Pneumo V452I device by a single technician. The best test among the consecutive three tests was accepted. FEV1, forced vital capacity (FVC), FEV1/FVC were measured according to American Thoracic Society criteria (16).
Analysis
Characteristics of the asthma patients were presented as median with range, or as mean with standard deviation according to the distribution of the parameters. In the analysis of statistical significance of selected characteristics of patients according to the AQLQ scores t-test, chi-square test, and Mann-Whitney U test were used where necessary.
Reliability analysis: Internal consistency and item-total score correlations were used for reliability analysis. Internal consistency was determined by Cronbach's alpha coefficient. Cronbach's alpha is recommended to be over 0.7 (17). The item and total score relationship was tested by Spearman correlation analysis.
Validity analysis: Validity of the Turkish version of the AQLQ was tested by using two different methods as Convergent Validity and Known Groups Method. Convergent validity refers that two instruments which evaluate same conditions will yield similar results or correlate highly. To assess convergent validity we used correlation (Spearman rank correlation coefficients) analysis for AQLQ and SF-36 domains. Known Groups Method is the ability of discriminating individuals with a known difference from others. It was tested by Student's t test and Mann-Whitney U test for LASS and pulmonary functions where appropriate.
The data were analyzed by SPSS 10.0 statistical package.
RESULTS
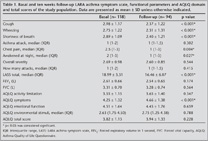
This study included 118 asthmatic patients aged between 18 and 55 years (mean age: 40.5 ? 10.2 years), 95 (79%) of whom were female. Thirty-eight percent of the patients had formal education of 5 years or less. Mild asthma was diagnosed in 73 (62%) of the patients while there was moderate-severe asthma in 45 (38%) according to GINA classification. The distribution of basal and follow-up asthma symptom scores, FEV1% predicted and AQLQ domains and total scores are shown in Table 1.
Reliability Analysis
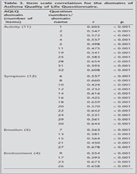
Cronbach- scores for activity (0.87), symptom (0.87), emotion (0.83) and environment (0.81) domains were found to be highly reliable (Table 2). Item versus domain correlations were determined to be high (0.44-0.83) and statistically significant. Item versus total score correlation coefficients ranged from 0.85 to 0.86 for activity domain; 0.85-0.89 for symptom domain and 0.77 to 0.87 for emotion domain and 0.75-0.77 for environment domain (Table 3).
Validity Analysis
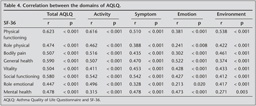
Convergent validity: At the basal evaluation, AQLQ activity domain, total score and SF-36 domain score correlations were in a range of fair degree (r= 0.315-0.626, p< 0.005). We found a little or fair correlation in other domains of AQLQ and SF36 (r= 0.241-0.542, p< 0.005) (Table 4). In the follow-up a similar relation was observed between AQLQ and SF-36 domain scores (r= 0.310-0.608, p< 0.005).
Known groups method: The AQLQ total and domain scores were found significantly different among asthma severity stages and symptom score levels determined by LASS in the first and follow-up visits (Table 5). However, we could not find any correlation between functional indices and the domains as well as the total score of AQLQ in both visits (p> 0.05 for each).
DISCUSSION
Asthma is a chronic respiratory disease associated with significant social, physical and psychological impact on daily activities of patients. HRQOL has been an important outcome measure in the management of asthma (18,19). Generic measures of HRQOL may be used in different settings, however they are not specific. Simple and feasible tools have been developed to assess asthma control. The performance of these instruments on psychometric tests among which good reliability and validity are considered the most important has to be established in countries other than the original form was developed (20). In the present study, we tested the validity and reliability of the Turkish version of AQLQ in a sample of Turkish asthmatic patients and confirmed that the Turkish version of AQLQ was reliable, valid and specific for the evaluation of the quality of life of asthma.
The reliability we observed by internal consistency of each scale showed that the use of the AQLQ subscales was feasible. The Cronbach's alpha coefficients were relatively high (0.746-0.864) and over the acceptable standards for reliability (17). We demonstrated that the homogeneity of the item scale correlations were in an adequate range. The patterns of correlation between the four AQLQ domains showed that the domains were related but measure separate aspects of asthma-specific quality of life. The results were similar to the findings of studies performed on different cultures (21,22).
The validity of quality of life questionnaire is usually evaluated on the basis of the association with other variables which it should theoretically be related (9). Therefore we looked for the correlations of AQLQ with a generic health care measurement SF-36. All domain scores and SF-36 domains were found significantly correlated in both first and second visits; however the relation was weak in the emotional function domain. Puhan et al. reported a fairly good internal consistency between AQLQ and SF-36 where they compared these two quality of life tools in 258 asthmatic patients (23). We also looked for known groups method and assessed the mean AQLQ total scores of patients according to disease severity and symptom score. We showed that as the asthma severity and symptoms increase, AQLQ decreased. Similarly, Sanju?s et al. showed significant differences in AQLQ scores in different severity indices and they found that the degree of dyspnea and all four domain and total scores of AQLQ were strongly correlated (21). It has also been demonstrated that patients with a severe form of disease had the lowest mean scores in all AQLQ domains and disease severity had a significant impact on the quality of life (22). The symptom scores were found to be significantly improved in the second visit in our study population. The AQLQ total score and activity limitation and environmental stimuli domains also improved, however the change was statistically in significant. The only significant change was observed in AQLQ symptom domain which also improved. This result suggests that specific AQLQ domains are prone to changes in the field that they evaluate and overall AQLQ score is a composite index measuring different indices. We could not show any correlation with pulmonary functions and AQLQ scores. A weak correlation was reported in some other studies (9,24). Functional parameters represent only a part of pathological process, therefore this result was not unexpected for us. Longitudinal validity was demonstrated by the correlation of the AQLQ mean scores and subgroup classifications according to LASS and asthma severity.
In conclusion, we concluded that the Turkish version of AQLQ is valid and reliable HRQOL tool for our population. Since doctors' and patients' perception of asthma severity is different, AQLQ can be used as an efficient indicator of asthmatic patients' health status.
CONFLICT of INTEREST
None declared.
REFERENCES
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001; 56: 880-7. [?zet] [PDF]
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt GH, Boulet LP, Haukioja A. Quality of life in asthma clinical trials: comparison of salmeterol and salbutamol. Am J Respir Crit Care Med 1995; 151: 66-70. [?zet]
- Boran P, Tokuc G, Pisgin B, Oktem S. Assessment of quality of life in asthmatic Turkish children. The Turkish Journal of Pediatrics 2008; 50: 18-22. [?zet]
- Juniper EF. Quality of life in adults and children with asthma and rhinitis. Allergy 1997; 52: 971-7. [?zet]
- Jones PW, the Nedocromil Sodium Quality of Life Study Group. Quality of life, symptoms and pulmonary function in asthma: longterm treatment with nedocromil sodium examined in a controlled multicentre trial. Eur Respir J 1994; 7: 55-62. [?zet]
- Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992; 47: 76-83. [?zet] [PDF]
- Hyland ME. The Living with Asthma Questionnaire. Respir Med 1991; 85 (Suppl B): 13-6. [?zet]
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321-7. [?zet]
- Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis 1993; 147: 832-8. [?zet]
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993; 46: 1417-32. [?zet]
- Hunt SM. Cross-cultural comparability of quality of life measures. Drug Info J 1993; 27: 395-400.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473-83. [?zet]
- Lara M, Sherbourne C, Duan N, Morales L, Gergen P, Brook RH. An English and Spanish pediatric asthma symptom scale. Med Care 2000; 38: 342-50. [?zet]
- Wood PR, Smith B, O'Donnell L. Quantifying asthma symptoms in adults: The Lara Asthma Symptom Scale. J Allergy Clin Immunol 2007; 120: 1368-72. [?zet]
- Ko?yi?it H, Aydemir ?, Fi?ek G, ?lmez N, Memi? A. K?sa Form-36'n?n (SF-36) T?rk?e versiyonunun g?venilirli?i ve ge?erlili?i. Romatizmal hastal??? olan bir grup hasta ile ?al??ma. ?la? ve Tedavi Dergisi 1999; 12: 102-6.
- American Thoracic Society Statement. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991; 144: 1202-18.
- Streiner DL, Norman GR. Health Measurement Scales. 2nd ed. New York: Oxford University Press, 1995.
- Guyatt GH, Feeny DH, Patrick DL. Measuring health related quality of life. Ann Intern Med 1993; 118: 622-9. [?zet] [Tam Metin] [PDF]
- Schmier JK, Chan KS, Leidy NK. The impact of asthma on health related quality of life. J Asthma 1998; 35: 585-97. [?zet]
- Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res 1993; 2: 451-9. [?zet]
- Tomic Spiric V, Bogic M, Jankovic S, Maksimovic N, Matovic Miljanovic S, Peric Popadic A, Raskovic S, Milic N. Assessment of the Asthma Quality of Life Questionnaire (AQLQ): Serbian translation. Croat Med J 2004; 45: 188-94. [?zet]
- Sanju?s C, Alonso J, Ferrer M, Curull V, Broquetas JM, Ant? JM. Adaptation of the Asthma Quality of Life Questionnaire to a second language preserves its critical properties: the Spanish version. J Clin Epidemiol 2001; 54: 182-9. [?zet]
- Puhan MA, Gaspoz JM, Bridevaux PO, Schindler C, Ackermann-Liebrich U, Rochat T, Gerbase MW. Comparing a disease-specific and a generic health-related quality of life instrument in subjects with asthma from the general population. Health Qual Life Outcomes 2008; 6: 15. [?zet] [Tam Metin] [PDF]
- Bousquet J, Knani J, Dhivert H, Richard A, Chicoye A, Ware JE Jr, Michel FB. Quality of life in asthma I. Internal consistency and validity of the SF-36 Questionnaire. Am J Respir Crit Care Med 1994; 149: 371-5. [?zet] [PDF]
Yaz??ma Adresi (Address for Correspondence):
Dr. Aylin ?zgen AlpaydIn,
Dokuz Eyl?l ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
?ZM?R - TURKEY
e-mail: aylin.ozgen@yahoo.com