Pediatrik uyku anketi T?rk?e formunun ge?erlilik ve
g?venilirli?i:
Uyku ile ili?kili solunum bozuklu?unun tahmininde bir ara?
Hasan Y?KSEL1, Ayhan S???T1, ?zge YILMAZ1, Ekrem KUTLUAY2
1 Celal Bayar ?niversitesi T?p Fak?ltesi, Pediatrik Allerji Bilim Dal?, Solunum Birimi, Manisa,
2 G?ney Carolina T?p ?niversitesi, N?robilimler B?l?m?, Charleston, Amerika Birle?ik Devletleri.
?ZET
Pediatrik
uyku anketi T?rk?e formunun ge?erlilik ve g?venilirli?i:
Uyku ile ili?kili solunum bozuklu?unun tahmininde bir ara?
Uyku ile ili?kili solunum bozukluklar?n?n tan? ve etkilerini tahmin edebilen bir anket kullan?lmas? hem tan? algoritmas?nda hem de tedavide maliyet-etkin olabilir. Bu nedenle, bu ?al??man?n amac?; Pediatrik Uyku Anketi (PSQ)?nin T?rk?e?ye ?evrilmesi ve T?rk?e anketin ge?erlilik ve g?venilirli?inin test edilmesidir. Uyku ile ili?kili solunum bozuklu?u ile uyumlu belirtileri olan 2-17 ya? aras?nda toplam 111 ?ocuk (59 erkek, 52 k?z) s?ras?yla ?al??maya al?nd?. ?al??maya al?nan t?m ?ocuklar?n ya? ve cinsiyet gibi demografik ?zellikleri kaydedildi. T?m ebeveynler, belirtilerin a??rl???, s?kl??? ve s?resi hakk?nda sorguland?. Son olarak, t?m ebeveynlere PSQ uyguland?. ?al??maya al?nan ?ocuklar?n ortalama ya?? 8.1 ? 3.4 y?l idi. Toplam PSQ puan? 0-0.95 aras?nda ve ortalama puan 0.35 ? 0.22 idi. Farkl? belirti s?kl??? bildiren ?ocuklar aras?nda toplam PSQ puanlar?n?n kar??la?t?r?lmas? PSQ puan?n?n belirti s?kl??? artt?k?a artt???n? g?sterdi (p< 0.001). Gruplar aras?nda t?m PSQ puanlar? anlaml? farkl?yd? (p< 0.05 t?m? i?in). Toplam PSQ puan?, hi? horlama rapor etmeyen ?ocuklar i?in 0.2 ? 0.5 iken, uyku boyunca horlayan ?ocuklar i?in 3.8 ? 0.5 idi (p< 0.001). PSQ?nun t?m alt puanlar? i?in Cronbach alfa de?erleri ba?ar?l?yd?. T?m sorular kendi alt skalalar? ile anlaml? koreleydi. PSQ?nun T?rk?e versiyonu, uyku ile ili?kili solunum bozuklu?unu d???nd?ren belirtileri olan T?rk ?ocuklar?n?n ilk de?erlendirmesinde kullan?labilecek g?venilir ve ge?erli bir ?l?ektir.
Anahtar Kelimeler: Pediatrik uyku anketi, uyku ile ili?kili solunum bozuklu?u, ?ocuk.
SUMMARY
Reliability and validity of the Turkish version of the pediatric sleep questionnaire: a tool for prediction of sleep related breathing disorder
Hasan Y?KSEL1, Ayhan S???T1, ?zge YILMAZ1, Ekrem KUTLUAY2
1 Department of Pediatric Allergy and Pulmonology, Faculty of Medicine, Celal Bayar University,
Manisa, Turkey,
2 Department of Neurosciences, Medical University of South Carolina, Charleston, United States of America.
Use of a questionnaire that predicts the diagnosis and influence of sleep related breathing disorder (SRBD) may be a cost-effective method to aid in both diagnostic algorithm and therapy. Therefore, the aim of this study was to adapt Pediatric Sleep Questionnaire (PSQ) into Turkish and to test the validity and reliability of the Turkish questionnaire. Total of 111 children (59 male, 52 female) aged 2 to 17 years who had symptoms suggestive of SRBD were enrolled consecutively. Demographic characteristics such as age and gender of all children enrolled in the study were recorded. All parents were questioned about symptom severity, frequency and duration. Lastly, PSQ was administered to all parents. Mean age of the children enrolled in the study was 8.1 ? 3.4 years. Total PSQ score ranged between 0 and 0.95 and mean score was 0.35 ? 0.22. Comparison of total PSQ scores between children reporting different symptom frequencies demonstrated that PSQ score increased as the symptom frequency increased (p< 0.001).There was a significant difference of all PSQ scores among the groups (p< 0.05 for all).Total PSQ score for children that did not report snoring was 0.2 ? 0.5 wile that for the ones who snore throughout sleep was 3.8 ? 0.5 (p< 0.001). Cronbach?s alpha values for all domains of PSQ were satisfactory. All items were significantly correlated with their corresponding scale. Turkish version of PSQ is a valid and reliable tool that may be used in the initial evaluation of Turkish children with symptoms suggestive of SRBD.
Key Words: Pediatric sleep questionnaire, sleep related breathing disorder, children.
Sleep related breathing disorders (SRBD) that constitute a spectrum of symptoms that range from habitual snoring to obstructive sleep apnea (OSA) have many adverse effects on many aspects of a child?s life and growth (1). The prevalence of habitual snoring has been reported to be in the range of 4-5% in our region and previous research has reported a prevalence of about 2% for OSA (2,3,4). Most common etiology of SRBD during childhood is adenotonsillar hypertrophy and allergic rhinitis but a pathology in neuromuscular tone has also been accused in etiology (5,6).
Gold standard for diagnosis and assessment of OSA in children is sleep laboratory based polysomnography (PSG) (1). However, PSG is an expensive technique that may not be available easily on many circumstances (7). Moreover, it is not thought to be very effective in predicting neurobehavioral morbidity due to SRBD because some features of OSA such as snoring that is not well quantified on standard PSG may be associated with cognitive and behavioral morbidity (8).
Pediatric Sleep Questionnaire (PSQ) developed and validated by Chervin et al. is a 22 item questionnaire which had been shown to have a sensitivity of 81% and specificity of 87% for SRBD (7). Considering the relative high prevalence of SRBD and the relative difficulties of obtaining PSG for all children suspected of having this disorder, use of a questionnaire that predicts the diagnosis and influence of SRBD may be a cost-effective method to aid in both diagnostic algorithm and therapy. Previous research with PSQ indicates that this questionnaire may be used with this aim (8,9,10).
Therefore, the aim of this study was to adapt PSQ into Turkish and to test the validity and reliability of the Turkish questionnaire.
MATERIALS? and METHODS
Subjects
Total of 111 children (59 male, 52 female) aged between 2 to 17 years who presented to the pediatric allergy and pulmonology outpatient department and who had symptoms suggestive of SRBD were enrolled in the study consecutively. Symptoms suggestive of SRBD were defined as the presence of snoring, difficulty breathing during sleep, mouth breathing during sleep or apnea.
Study Design
Demographic characteristics such as age and gender of all children enrolled in the study were recorded. All parents were questioned about symptom severity, frequency and duration. Symptom severity was assessed by asking the parents to classify the breathing of their child during sleep as loud breathing/snoring, difficulty breathing or apnea during sleep. Snoring frequency was classified as 1-3 nights a week, 4-6 nights a week and every night. Moreover, symptom duration was classified as snoring for less than half of the night, snoring for more than half of the night and snoring all night long. Lastly, PSQ was administered to all parents.
Pediatric Sleep Questionnaire: Sleep Related
Breathing Disorder Scale
PSQ: SRBD scale is a questionnaire that can be administered to parents of children aged 2-18 years and is composed of 22 items that question frequency and severity of snoring during sleep, apnea at night during sleep, breathing difficulty during sleep, daytime sleepiness, attention deficit, hyperactivity and other pediatric obstructive sleep apnea symptoms. Responses to the items are as ?yes?, ?no? and ?I don?t know? which are scored as 1, 0 and missing respectively. Four of the items are about sleepiness, four about snoring and six about attention deficit/hyperactivity disorder defined according to the diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (11). Total score of PSQ is the mean of the scores of all items excluding the missing items. Most effective cut-off value for pediatric obstructive sleep apnea has been defined as 0.33.
Adaptation into Turkish
Turkish adaptation included four main steps:
1. Forward translation: Two independent translators translated the questionnaire into Turkish.
2. Consensus forward translation: Two independent pediatricians fluent in English and Turkish developed one consensus Turkish questionnaire from the two translations.
3. Backtranslation: This consensus forward version was back translated into English by two independent translators who have not seen the original questionnaire and the backward version and the original text were compared by an independent supervisor.
4. Cognitive debriefing: The questionnaire was administered to ten patients who were not included in the field testing analysis.
Statistical Analysis
The data was analyzed by SPSS 13.0 (Chicago, IL) statistical package. p values < 0.05 were regarded as statistically significant. The statistical analysis consisted of reliability and validity analysis.
Internal consistency and item-total score correlations were used for reliability analysis. Internal consistency was tested using Cronbach alpha for every sub-scale of the instrument since every sub-scale represents a different concept for the patients. The item score and total score relationships were explored by Pearson?s correlation analysis.
Validity of the Turkish version of the PAQLQ was tested by construct validity analysis. Construct validity was tested by using known group method tested by ANOVA analysis.
RESULTS
Sociodemographic Characteristics
Mean age of the children enrolled in the study was 8.1 ? 3.4 and ranged between 2 and 17 years. Among all children 53.2% were males and 46.8% were females. Tonsil hypertrophy was present in 16.2% and 13.5% had allergic rhinitis (Table 1).
Distribution of PSQ Scores
Total PSQ score ranged between 0 and 0.95 and mean score was 0.35 ? 0.22. Snoring score ranged between 0 and 1.25 and mean was 0.26 ? 0.36. Mean sleepiness and inattention domain scores were 0.26 ? 0.28 and 0.51 ? 0.36 respectively.
Validity of the Turkish PSQ
Comparison of total PSQ scores between children reporting different symptom frequencies demonstrated that PSQ score increased as the symptom frequency increased (p< 0.001). Similarly, snoring and inattention scores were significantly higher in children for whom higher symptom frequency was reported (p< 0.001 and p= 0.007 respectively). However, sleepiness scale was not found to be significantly different between different symptom frequency groups (Table 2).
When children were grouped according to symptom severity, there was a significant difference of all PSQ scores among the groups (p< 0.05 for all). Total score was calculated to be 0.2 ? 0.6 in children who did not report severe symptoms while it was calculated as 3.6 ? 0.9 in children with apnea during sleep (p< 0.001) (Table 2).
Another grouping category to test for known group analysis was the symptom duration. It was demonstated that total, snoring and inattention scores were all significantly different scores while sleepiness score was not. Total PSQ score for children that did not report snoring was 0.2 ? 0.5 wile that for the ones who snore throughout sleep was 3.8 ? 0.5 (p< 0.001) (Table 2).
Reliability of the Turkish PSQ
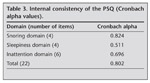
Cronbach?s alpha value for snoring domain was 0.824 (Table 3). Item scale correlations for snoring domain were significant for all items. Pearson?s correlation coefficient for item ?Does your child snore more than half the time?? was 0.86 while that of ?Does your child always snore? was 0.82. The items ?Does your child snore loudly? and ?Does your child have ?heavy? or loud breathing?? had correlation coefficients of 0.83 and 0.75 respectively.
For sleepiness domain, Cronbach?s alpha value was 0.511 (Table 3). When correlated with the total sleepiness score, items ?Does your child wake up feeling unrefreshed in the morning?? and ?Does your child have a problem with sleepiness during the day?? had pearson?s correlation coefficients of 0.73 and 0.59 respectively. Similarly, the items ?Has a teacher or other supervisor commented that your child appears sleepy during the day?? and ?Is it hard to wake your child up in the morning?? were significantly correlated with the total domain score (r= 0.49 and r= 0.69 respectively).
Cronbach?s alpha value for inattention domain was 0.696 (Table 3). Item scale correlations for the items ?does not seem to listen when spoken directly?, ? has difficulty organizing tasks and activities? and ?Is easily distracted by extraneous stimuli? were 0.74, 0.56 and 0.65 respectively. Similarly items ?Fidgets with hands or feet or squirms in seat?, ?Is ?on the go? or often acts as if ?driven by a motor? and ?nterrupts or intrudes on others (eg., butts into conversations or games)? were all significantly correlated with the inattention domain score (r= 0.62 for all).
DISCUSSION
Sleep disordered breathing disorders that constitute a wide spectrum of symptoms ranging from habitual snoring to OSA have prevalences reported as low as 2% for OSA and as high as 27.6% for habitual snoring (1,2,3,4,12). SRBD is clinically and epidemiologically important because it influences not only growth but is also associated with deterioration in quality of life and neurocognitive deficits as well as emotional immaturiy in children and treatment of SRBD with adenotonsillectomy was shown to improve quality of life and behavior of children with OSA (13,14,17). Moreover, children with SRBD during kindergarten years were found to have lower school performance during the first year of sleep (15). Poor sleep quality leading to neurocognitive developmental defects and deficits in verbal abilities is a cause for lower academic achievement in children and adolescents with OSA (14,15,18). Gold standard for diagnosis and assessment of OSA in children is sleep laboratory based polysomnography (PSG) (1). However, PSG is an expensive technique that may not be available easily on many circumstances therefore a questionnaire based initial evaluation may aid in patient selection for PSG.
PSQ developed and validated by Chervin et al. is a 22-item questionnaire which had been shown to have a sensitivity of 81% and specificity of 87% for SRBD (7). The responses to the scale depend on subjective parent impressions in part and it is thought to reflect the impact of disruptive behavior on affected families (8). Therefore, validation of the questionnaire was performed based on known groups method by questioning symptom severity, duration and frequency in our study. It was shown that both total and all domain scores including snoring, sleepiness and inattention were significantly different among different severity groups. This is an expected results since both the severity and scale itself are the subjective reports of parents. When the scores were compared on the basis of frequency and duration, it was observed that total score as well as the snoring and inattention scores differed significantly but sleepiness domain score did not. This might be due to the fact that sleepiness is a more indirect consequence of SRBD so although severity had an influence on sleepiness, frequency and duration did not.
Reliability analysis of the Turkish PSQ consisted of Cronbach alpha calculations and item-scale correlations. According to the general guidelines suggested by Colton, correlations ranging 0.00 to 0.25 indicate little or no relationship; those from 0.25 to 0.50 suggest a fair degree of relationship; values of 0.50 to 0.75 are moderate to good; and values above 0.75 are considered good to excellent. Item-scale correlations for the Turkish PSQ were all above 0.50 indicating moderate to good correlation indicating a successful internal consistency of the adapted scale.
All Cronbach alpha values were above 0.50 for the PSQ domains. Considering that reliability is a measure of a scale?s stability, these results indicate that Turkish questionnaire is a valid tool that may be used for initial evaluation of children suspected of having SRBD (19).
Main limitations of this study were absence of PSG evaluations for all children since PSG is a gold standard for diagnosis of SRBD. However, the validity and reliability of the original questionnaire had already been performed by Chervin et al. by comparisons with PSG results (7,8). Therefore, we did not try to reproduce their findings but only evaluated for the validity and reliability of the Turkish questionnaire. Therefore, absence of PSG may not be considered a major draw-back.
In conclusion, Turkish version of PSQ is a valid and reliable tool that may be used in the initial evaluation of Turkish children with symptoms suggestive of SRBD.
ACKNOWLEDGEMENTS
We thank Prof. Ron Chervin for his generous help in the Turkish adaptation of PSQ as well as the validation study.
CONFLICT of INTEREST
None declared.
REFERENCES
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008; 5: 242-52. [?zet] [Tam Metin] [PDF]
- Sogut A, Yilmaz O, Dinc G, Yuksel H. Prevalence of habitual snoring and symptoms of sleep-disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol. 2009; 73: 1769-73. [?zet]
- Yilmaz O, Dinc G, Sogut A, Aktulun S, Arslan B,? Kocacan M, et al. Prevalence of habitual snoring in Aegean region of Turkey and associated risk factors. Turk Arch Ped 2010 (in press).
- Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, et al. Sleep-disordered breathing in 3680 Greek children. Pediatr Pulmonol 2004; 37: 499-509. [?zet]
- Wildhaber JH, Moeller A. Sleep and respiration in children: time to wake up! Swiss Med Wkly 2007; 137: 689-94. [?zet] [PDF]
- Sogut A, Yilmaz O, Dinc G, Yuksel H. Prevalence of habitual snoring and symptoms of sleep-disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol 2009.
- Chervin RD, Hedger KM, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000; 1: 21-32. [?zet]
- Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007; 133: 216-22. [?zet] [Tam Metin] [PDF]
- Desager KN, Nelen V, Weyler JJ, De Backer WA. Sleep disturbance and daytime symptoms in wheezing school-aged children. J Sleep Res 2005; 14: 77-82. [?zet] [PDF]
- Chervin RD, Weatherly RA, Ruzicka DL, Burns JW, Giordani BJ, Dillon JE, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep 2006; 29: 495-503. [?zet] [Tam Metin] [PDF]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association, 1994.
- Ng DK, Chan C, Chow AS, Chow P, Kwok K. Childhood sleep-disordered breathing and its implications for cardiac and vascular diseases. J Paediatr Child Health 2005; 41: 640-6. [?zet]
- Mitchell RB, Boss EF. Pediatric obstructive sleep apnea in obese and normal-weight children: impact of adenotonsillectomy on quality-of-life and behavior. Dev Neuropsychol 2009; 34: 650-61. [?zet]
- Honaker SM, Gozal D, Bennett J, Capdevila OS, Spruyt K. Sleep-disordered breathing and verbal skills in school-aged community children. Dev Neuropsychol 2009; 34: 588-600. [?zet]
- Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Sleep disturbances are associated with reduced school achievements in first-grade pupils. Dev Neuropsychol 2009; 34: 574-87. [?zet]
- Marcus CL. Sleep-disordered breathing in children. Curr Opin Pediatr 2000; 12: 208-12. [?zet]
- Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 2004; 114: 805-16. [?zet] [Tam Metin] [PDF]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health 2010; 46: 124-32. [?zet]
- Rutishauser C, Sawyer SM, Bowes G. Quality of life assessment in children and adolescents with asthma. Eur Respir J 1998; 12: 486-94. [?zet] [PDF]
Yaz??ma Adresi (Address for Correspondence):
Dr. Hasan Y?KSEL,
Celal Bayar ?niversitesi T?p Fak?ltesi,
Pediatrik Allerji Bilim Dal? ve Solunum Birimi,
MAN?SA - TURKEY
e-mail: oyilmaz_76@hotmail.com