T?berk?lin cilt testi pozitif saptanan ?ocuklar?n ?zellikleri
Arzu
BABAY???T HOCAO?LU1, Duygu ?LMEZ ERGE1, ?zden ANAL2,
Balahan MAKAY3, Nevin UZUNER1,
?zkan KARAMAN1
1 Dokuz Eyl?l ?niversitesi T?p Fak?ltesi, ?ocuk Sa?l??? ve Hastal?klar? Anabilim Dal?, ?ocuk Allerji Bilim Dal?, ?zmir,
2 Dokuz Eyl?l ?niversitesi T?p Fak?ltesi, ?ocuk Sa?l??? ve Hastal?klar? Anabilim Dal?, ?ocuk ?mm?noloji Bilim Dal?,
?zmir,
3 Dokuz Eyl?l ?niversitesi T?p Fak?ltesi, ?ocuk Sa?l??? ve Hastal?klar? Anabilim Dal?, ?zmir.
?ZET
T?berk?lin cilt testi pozitif saptanan ?ocuklar?n ?zellikleri
Bu ?al??man?n amac?; pozitif t?berk?lin cilt testi ile latent t?berk?loz tan?s? konulan ?ocuk hastalar i?in potansiyel risk fakt?rlerini de?erlendirmektir. Latent t?berk?loz tan?s? ile izlenmekte olan ?ocuk hastalar retrospektif olarak ?al??maya dahil edildi. Hastalar?n atopi ?yk?s?, ge?irilmi? solunum yolu infeksiyonlar?, t?berk?loz ve atopi a??s?ndan aile ?yk?s?, BCG a?? say?s?, fizik muayene bulgular? ve laboratuvar sonu?lar?n? i?eren demografik ?zellikleri hastalar?n dosyalar?ndan kaydedildi. T?berk?lin cilt testi pozitifli?i saptanan 81 (51 erkek, 30 k?z) ?ocuk bu ?al??ma kapsam?nda de?erlendirildi. Hastalar?n ortalama ya?lar? 8.00 ? 4.00 idi. Sadece 13 (%16) ?ocu?un aktif t?berk?lozlu birey ile temas ?yk?s? mevcuttu. Hastalar?n ya?lar?, BCG a?? ve skar say?s?n?n t?berk?lin cilt testi reaksiyon b?y?kl???n? istatistiksel olarak ?nemli oranda etkilemekte oldu?u g?sterildi. T?berk?lin cilt testi reaksiyon b?y?kl???, son doz BCG a??s?ndan sonra ge?en s?re, ailede aktif t?berk?loz ve t?berk?lin cilt testi pozitif birey varl???, sigara maruziyeti, ailenin birey say?s? ve allerjik solunum yolu hastal??? varl??? ile etkilenmedi?i tespit edildi. Hastan?n ya??, BCG a?? ve skar say?s?n?n ?ocukluk ?a??nda t?berk?lin cilt testi sonu?lar?n? ?nemli ?l??de etkiledi?i g?sterildi. Bu durum, latent t?berk?loz tan?s? koymada ve profilaktik tedavi ba?lanmas? karar?nda g??l?klere neden olabilir. Bu ?al??man?n sonu?lar?, latent t?berk?loz tan?s? koymak i?in daha y?ksek maliyetli ve sofistike laboratuvar gerektiren yeni geli?tirilmi?, daha do?ru ve g?venilir sonu?lar veren in vitro testlerin kullan?labilece?ini d???nd?rmektedir.
Anahtar Kelimeler: T?berk?loz, t?berk?lin testi, ?ocuk.
SUMMARY
Characteristics of children with positive tuberculin skin test
Arzu
BABAY???T HOCAO?LU1, Duygu ?LMEZ ERGE1, ?zden ANAL2,
Balahan MAKAY3, Nevin UZUNER1,
?zkan KARAMAN1
1 Department of Children Health and Diseases, Division of Children Allergy, Faculty of Medicine,
Dokuz Eylul University, Izmir, Turkey,
2 Department of Children Health and Diseases, Division of Children Immunology, Faculty of Medicine,
Dokuz Eylul University, Izmir, Turkey,
3 Department of Children Health and Diseases, Faculty of Medicine, Dokuz Eylul University, Izmir, Turkey.
The aim of the study was to define the characteristics of children with latent tuberculosis diagnosed with positive tuberculin skin test (TST) and evaluate potential risk factors in children with positive TST. Children followed with the diagnosis of latent tuberculosis infection were included in the study retrospectively. Demographic characteristics of patients including history of atopy, respiratory infections, family history of tuberculosis and atopy, number of BCG vaccinations, findings of physical examination and laboratory data were extracted from patient's file. Eighty-one children (51 male, 30 female) who had positive TST were retrospectively evaluated in the study. Mean age of the patients was 8.00 ? 4.00 years. Only 13 (16%) of the children had contact with a case who had active tuberculosis. It was shown that the age of the patients, number of BCG scars and BCG vaccination significantly affected TST reaction size. TST size was not affected with time passed after last dose of BCG vaccination, family history of tuberculosis, presence of TST positive case in the family, exposure to cigarette smoke, number of household family members and presence of respiratory allergic disease. The patient's age, numbers of BCG vaccination and BCG scars significantly affect TST results in childhood. This may cause difficulty in diagnosing latent tuberculosis infection and in decision of initiating prophylactic treatment. The results of this study may show that recently developed, more accurate and convenient in vitro tests that they have higher costs and require sophisticated laboratory, can be used to diagnose latent tuberculosis.
Key Words: Tuberculosis, tuberculin test, child.
Tuberculosis (TB) is still a significant health problem worldwide (1). The disease has two stages in pathogenesis; latent infection precedes the appearance of clinical disease. The latent phase, in which the individual acquires the infection, is referred to as tuberculosis infection (2). Tuberculin skin testing (TST) is being used for diagnosing and estimating the prevalence of TB infection, for identifying individuals who need prophylactic treatment and for tracing of TB transmission to contacts of cases with active TB (3).
Recurrent respiratory problems in a child require careful clinical evaluation for mainly chronic pulmonary infections such as tuberculosis and allergic airway diseases. The epidemiological relation between mycobacterial infection and atopic disease in humans has still not been clarified (4,5,6,7).
The aim of this study was to determine the characteristics of children with latent TB diagnosed with positive TST, to evaluate the potential factors leading to positive TST and to investigate the relationship between positivity of TST and childhood respiratory allergic diseases.
MATERIALS and METHODS
Children who have been followed with diagnosis of latent TB and received prophylactic treatment in Pediatric Outpatient Clinic of Dokuz Eylul University Hospital, Izmir, Turkey, between 2005 and 2007 were included in the study retrospectively. Within the last 12 months prior to diagnosis, none of these children had a tuberculin skin test, or BCG vaccination.
The following information was obtained from the patient's files: demographic characteristics, history of atopy, childhood respiratory infections, family history of tuberculosis and atopy, exposure to cigarette smoke, number of family members, number of BCG vaccinations and BCG scars, physical examination and radiological findings.
A standard method (Mantoux) was used for administration of TST to all of the patients. A steel needle of 27 gauge attached to a 1 mL syringe was used to administer 5 tuberculin units (0.1 mL) of protein purified derivative (PPD) solution intradermally on the volar surface of the forearm in the outpatient clinic. Patients were explained not to wash or scratch the TST area. The reactions were read after 72 hours by measuring the two diameters of the induration, horizontally and vertically with the margins marked by a ball-point pen. The mean of two diameters was used for the statistical analysis. The test was accepted as positive for latent TB infection if the reactive induration was measured ≥ 15 mm. Active TB disease was ruled out in each patient.
In the evaluation of children admitted with symptoms of respiratory allergic diseases, epidermal skin prick tests were applied with common allergens using Allergopharma prick tests and evaluated according to Aas and Berlin criteria (8). Sensitization to house dust mites, grasses, tree pollens, cereals, wild grass pollens, animal danders, moulds, cockroach, food and latex were evaluated with prick tests. Serum total IgE was determined by ELISA. Pulmonary function tests were performed if the child had chronic cough and/or symptoms of bronchial asthma.
Statistical Analysis
SPSS 11.0 statistical package for Windows was used throughout the study. Results were shown as mean ? SD. To perform statistical analysis, the patients were divided into two groups; the patient who had respiratory allergic diseases (atopic group) and who had no respiratory allergic disorders (non-atopic group). They were also studied in two groups according to their TST size. Chi-square analyses were conducted among the subgroups to test for statistically significant differences. A p< 0.05 was considered statistically significant.
RESULTS
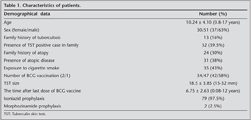
Eighty-one children who had positive TST were retrospectively included the study. 51 were male (63%) and 30 were female (37%). Mean age of the patients was 8.00 ? 4.00 years (range: 0-17 years). The demographic data of patients is shown in Table 1.
Thirty-two patients (39.5%) had no symptoms and were admitted during family screening for TB. 28 children (34.5%) were initially admitted with chronic cough, 21 (26%) with recurrent respiratory tract infections.
On chest examination, crackles were heard in 2.5%, and ronchi, in 18.5% of children. The physical examination of 64 patients (79%) was unremarkable.
Family screening revealed that 13 children (16%) had contact with a case who had active TB. 32 of the patients (39.5%) had at least one TST positive family member.
Thirty-one (38%) children were able to perform pulmonary function tests. Twelve (38%) had obstructive lung changes whereas 19 (61%) had results within normal ranges.
All patients had chest radiograms. High resolution computerized tomography (HRCT) was performed in 1/3 of the patients (27 patients) who had chronic respiratory symptoms. Radiographic evaluation of all patients were unremarkable.
When the patients were divided into two groups according to their TST size [15 ≤ TST < 20 mm (n= 51) and TST ≥ 20 mm (n= 30)], the age of the patient, number of BCG vaccination and scars of BCG vaccination significantly affected TST reaction size. Family history of TB, presence of TST positive case in household, exposure to cigarette smoke, number of family members, radiological findings and presence of respiratory allergic disease in the patient were not statistically different between the two groups (Table 2). The mean time after last dose of BCG vaccine was 6.75 ? 2.63 years (0.08-12 years) and no correlation was found between the time after the last dose of vaccination and TST results.
In the study group 31 patients had also allergic respiratory diseases including bronchial asthma (100%) and both asthma and allergic rhinitis (50%). Total IgE levels were between 0-606 IU/mL (103.17 ? 133.9 IU/mL) in the whole study population. The differences between allergic and non-allergic groups are summarized in Table 3. The size of TST was not statistically different between allergic and non-allergic groups.
DISCUSSION
Pediatric TB should be accepted as a public-health emergency, because young children have a much higher risk of developing severe and fatal disease than adult cases (1,9,10,11,12). The Turkish Ministry of Health started a routine national immunization programme in order to prevent TB since 1953. In 1990's two doses of BCG were administered in children, the first within 2 months of birth and the second at the age of 7 years, and in 2006, a single dose is started to be administered at 2 months of age.
TST is an old, inexpensive and easily administered test which maintains its significance in determining infection with Mycobacterium tuberculosis, particularly in children (13). TST reactivity becomes apparent within 3-6 weeks following M. tuberculosis infection and may remain positive for many years (14,15). This test can accurately identify individuals in need of prophylactic treatment and investigate contacts of active TB patients (13). In the current study, the demographic data and potential risk factors of children who had positive TST reaction and the association between TST positivity and respiratory allergic diseases were investigated. It is accepted that positive skin tests with indurations of ≥ 15 mm are more likely to be the result of TB infection than of BCG vaccination (16). Because of this reason, the cut-off level used in the current study that defines a positive TST is believed to have increased the specificity of the TST in the study population.
In this study, the age of the patient, number of BCG vaccination and scars of BCG vaccination significantly affected TST reaction size. Consistent with previous findings, a trend of increasing TST positivity with increasing age was observed in this study (17). While data regarding the influence of BCG vaccination on TST results is conflicting, it has been shown that BCG may induce cross-reactions for a prolonged period after vaccination (18). Hence, the recommended cut-off value of ≥ 15 mm skin induration on TST was used as an indicator of M. tuberculosis infection in this study. A meta-analysis showed that immunization with BCG increased the risk of a positive skin test result although several studies have shown that the skin test reaction wanes with time after BCG vaccination (16,19,20,21,22). The results showed that the number of BCG vaccination and BCG scars had statistically significant effects on TST reaction size.
Sex differences in the epidemiology of TB are well known and have been extensively documented. Lower rates in females have been usually attributed to gender differences in TB epidemiology (23). Men in the community control households had twice the risk of positive TST compared with women (3). The results of many studies showed that men had a higher prevalence of positive TST reactions than women consistent with our findings (21,24,25). In this study, two third of the study population who had positive TST were boys.
Epidemiologic studies have demonstrated that risk of TB is increased among close contacts of sputum smear-positive patients (26,27). High rates of transmission were found within households of smear-positive TB patients living in areas of high prevalence (28). However, it has been suggested that the majority of cases are acquired from an unknown non-intimate contacts (29). In this study, only 16% of children had close contact with smear-positive TB cases and nearly 40% had at least one TST positive case within household. Most of the TST positive cases (53.1%) detected with family screening were the siblings of the patients. Crowding, higher number of family members and the intensity of exposure have previously been reported as important risk factors for skin test positivity (30,31,32). The number of family members had no apparent effect on skin test responses in this study group.
Passive smoking may have harmful effects in children because their respiratory systems are still developing? but there is only a few studies investigating the association between environmental tobacco smoke exposure (passive smoking) and acquiring Mycobacterium tuberculosis infection (33,34). Although Kuemmerer and Comstock reported that TST reactions were larger in children in whom both parents smoked, there was no relationship between passive smoking and TST reactivity in the current study (35).
Conflicting results are found in the literature about TST reactivity and allergic diseases. Some studies suggested that there was no relationship between the tuberculin response and allergic disease (36,37,38). Shirakawa et al. reported that asthma incidence, serum IgE, and T helper 2 type cytokines such as Interleukin (IL)-4, IL-10, and IL-13 were lower in children with positive TST compared with those of children with negative results (5). A previous study from Turkey showed larger TST reactivity in 106 allergic children vaccinated with BCG than non-allergic children (39). There was no relationship between the TST induration size and atopic state of patients in this study.
Treatment of latent TB infection is important to prevent future disease activation. WHO guidelines recommend all children under 5 years in close contact with an infectious case receive 6 months isoniazid once active disease has been excluded. The currently preferred regimen is 6-9 months daily isoniazid which has been proven to reduce the TB risk in exposed children more than 90% if completed properly (40,41). In our study population, 79 children (97.5%) received isoniazid for 6 months. Only 2 children whose parents had TB resistant to isoniazid and rifampicin received morphozinamide.
In conclusion; the results of this study showed that the age of the child, numbers of BCG vaccination and BCG scars should be carefully considered while ruling out TB infection especially in school-age children with prolonged respiratory symptoms. The results of this study showed that although they have higher costs and require sophisticated laboratory, recently developed, more accurate and convenient in vitro tests may be used to diagnose latent TB.
CONFLICT of INTEREST
None declared.
REFERENCES
- Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995; 273: 220-6. [?zet]
- Enarson DA, Ait-Khaled N. Tuberculosis. In: Annesi- Maesano I, Gulsvik A, Viegi G (eds). Respiratory Epidemiology in Europe. Huddersfield: The Charlesworth Group, 2000: 67-91.
- Gustafson P, Lisse I, Gomes V, Vieira CS, Lienhardt C, Naucl?r A, et al. Risk factors for positive tuberculin skin test in Guinea-Bissau. Epidemiology 2007; 18: 340-7. [?zet]
- Von Mutius E, Pearce N, Beasley R, Cheng S, von Ehrenstein O, Bj?rkst?n B, et al. International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis and eczema. Thorax 2000; 55: 449-53. [?zet] [PDF]
- Shirakawa T, Enomoto T, Shimazu S, Hopkin J. The inverse association between tuberculin responses and atopic disorder. Science 1997; 275: 77-9. [?zet]
- Von Hertzen L, Klaukka T, Mattila H, Haahtela T. Mycobacterium tuberculosis infection and the subsequent development of asthma and allergic conditions. J Allergy Clin Immunol 1999; 104: 1211-4. [?zet]
- Omenaas E, Jentoft H, Vollmer W, Buist A, Gulsvik A. Absence of relationship between tuberculin reactivity and atopy in BCG vaccinated adults. Thorax 2000; 5: 454-8. [?zet] [PDF]
- Aas K, Belin L. Standardization of diagnostic work in allergy. Acta Allergologica 1972; 27: 439-68.
- Feja K, Saiman L.Tuberculosis in children. Clin Chest Med 2005; 26: 295-312. [?zet]
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004; 8: 636-47. [?zet]
- Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. J Infect 2004; 48: 13-22. [?zet]
- Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990-2000. Bull World Health Organ 1994; 72: 213-20. [?zet] [PDF]
- Enarson DA. Children and the global tuberculosis situation. Paediatr Respir Rev 2004; 5(Suppl A): S143-5. [?zet]
- Committee of Infectious Disease. Screening for tuberculosis in infants and children. Pediatrics 1994; 93 :131-4.
- Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis 2003; 3: 624-32. [?zet]
- Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 2002; 57: 804-9. [?zet] [PDF]
- Bener A, Uduman S, Bin-Othman SA. Factors associated with tuberculin reactivity among children in United Arab Emirates. Resp Med 1996; 90: 89-94. [?zet]
- Comstock GW, Livesay VT, Woolpert SF. Evaluation of BCG vaccination among Puerto Rican children. Am J Public Health 1974; 64: 293-1. [PDF]
- Saiman L, San Gabriel P, Schulte J, Vargas MP, Kenyon T, Onorato I. Risk factors for latent tuberculosis infection among children in New York City. Pediatrics 2001; 107: 999-1003. [?zet]
- Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis 1999; 3: 962-75. [?zet]
- Jentoft HF, Omenaas E, Eide GE, Gulsvik A. Tuberculin reactivity: prevalence and predictors in BCG-vaccinated young Norwegian adults. Respir Med 2002; 96: 1033-9. [?zet]
- Snider DE Jr. Bacille Calmette-Guerin vaccinations and tuberculin skin tests. JAMA 1985; 253: 3438-9.
- Borgdorff MW, Nagelkerke NJD, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis 2000; 4: 123-32. [?zet]
- Lienhardt C, Fielding K, Sillah J, Tunkara A, Donkor S, Manneh K, et al. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med 2003; 168: 448-55. [?zet] [Tam Metin] [PDF]
- Bener A, Abdullah AK. Reaction to tuberculin testing in Saudi Arabia. Indian J Public Health 1993; 37: 105-10. [?zet]
- Narain R, Nair SS, Ros GR, Chandrasekhar P. Distribution of tuberculous infection and disease among households in a rural community. Bull World Health Organ 1996; 34: 639-54. [?zet] [PDF]
- Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: the effects of chemotherapy. Tubercle 1976; 57: 275-9. [?zet]
- Beyers N, Gie RP, Schaaf HS, Van Zyl S, Talent JM, Nel ED, et al. A prospective evaluation of children under the age of 5 years living in the same household as adults with recently diagnosed pulmonary tuberculosis. Int J Tuberc Lung Dis 1997; 1: 31-7. [?zet]
- Madico G, Gilman RH, Checkley W, Cabrera L, Kohlstadt I, Kacena K, et al. Community infection ratio as an indicator for tuberculosis control. Lancet 1995; 345: 416-9. [?zet]
- Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc 1975; 50: 90-106.
- Almeida LM, Barbieri MA, Da Paixao AC, Cuevas LE. Use of purified protein derivative to assess the risk of infection in children in close contact with adults with tuberculosis in a population with high Calmette-Guerin bacillus coverage. Pediatr Infect Dis J 2001; 20: 1061-5. [?zet]
- Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc 1954; 69: 724-32. [?zet] [PDF]
- Cook DG, Strachan DP. Health effects of passive smoking: summary of effects of parental smoking on the respiratoryhealth of children and implications for research. Thorax 1999; 54: 357-66.
- den Boon S, Verver S, Marais BJ, Enarson DA, Lombard CJ, Bateman ED, et al. Association between passive smoking and infection with Mycobacterium tuberculosis in children. Pediatrics 2007; 119: 734-9. [?zet] [Tam Metin] [PDF]
- Kuemmerer JM, Comstock GW. Sociologic concomitants of tuberculin sensitivity. Am Rev Respir Dis 1967; 96: 885-92.
- Stannegard IL, Larsson LO, Wennergren G, Strannegard O?. Prevalence of allergy in children in relation to prior BCG vaccination and infection with atypical mycobacteria. Allergy 1998; 53: 249-54. [?zet]
- Omenaas E, Jentoft HT, VollmerWM, Buist AS, Gulsvik A. Absence of relationship between tuberculin reactivity and atopy in BCG vaccinated young adults. Thorax 2000; 55: 454-8. [?zet] [PDF]
- Yilmaz M, Bingol G, Altintas D, Kendirli SG. Correlation between atopic diseases and tuberculin responses. Allergy 2000; 55: 664-7. [?zet] [Tam Metin] [PDF]
- Ozmen S, Tomac N, Uysal A, Arslan Z, Kuyucu N, Yoney A. Tuberculin responses in children with allergic diseases. Allergy 2002; 57: 1059-66. [?zet] [Tam Metin] [PDF]
- Hsu KH. Thirty years after isoniazid. Its impact on tuberculosis in children and adolescents. JAMA 1984; 251: 1283-5. [?zet]
- Lobue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology 2010; 15: 603-22. [?zet] [Tam Metin] [PDF]
Yaz??ma Adresi (Address for Correspondence):
Dr. Arzu BABAY???T HOCAO?LU,
Dokuz Eyl?l ?niversitesi T?p Fak?ltesi,
?ocuk Sa?l??? ve Hastal?klar? Anabilim Dal?,
?ocuk Allerji Bilim Dal?,
35340 ?ZM?R - TURKEY
e-mail: arbabayigit@yahoo.com