Hemodiyaliz hastalar?nda latent t?berk?loz infeksiyonu taramas?nda QuantiFERON-TB Gold test
Hayriye
SAYARLIO?LU1, Mustafa G?L2, Canan EREN DA?LI3,
Ekrem DO?AN1, Murat ?AH?N4,
Mehmet Ali U?AR4, Nurhan K?KSAL3, Mehmet SAYARLIO?LU4,
M?mtaz Kerim TAHTA5
1 Kahramanmara? S?t?? ?mam ?niversitesi T?p Fak?ltesi, ?? Hastal?klar? Anabilim Dal?, Nefroloji Bilim Dal?,
Kahramanmara?,
2 Kahramanmara? S?t?? ?mam ?niversitesi T?p Fak?ltesi, Mikrobiyoloji Anabilim Dal?, Kahramanmara?,
3 Kahramanmara? S?t?? ?mam ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Kahramanmara?,
4 Kahramanmara? S?t?? ?mam ?niversitesi T?p Fak?ltesi, ?? Hastal?klar? Anabilim Dal?, Kahramanmara?,
5 SB Kahramanmara? Devlet Hastanesi, ?? Hastal?klar? Klini?i, Kahramanmara?.
?ZET
Hemodiyaliz hastalar?nda latent t?berk?loz infeksiyonu taramas?nda QuantiFERON-TB Gold test
Genel pop?lasyonla kar??la?t?r?ld???nda hemodiyaliz hastalar?nda latent t?berk?loz infeksiyonu (LTB?) riski artm??t?r. Hemodiyaliz hastalar?nda LTB? ara?t?r?lmas?nda QuantiFERON-TB Gold (QFT-G) test t?berk?loz cilt testi (TCT)?nden daha umut vericidir. ?al??man?n amac? hemodiyaliz hastalar?ndaki LTB? tan?s?nda QFT-G?nin TCT?den daha hassas olup olmad???n? belirlemektir. LTB? i?in TCT ve QFT-G ile 89 hemodiyaliz hastas? de?erlendirildi. T?m hastalarda QFT-G i?in kan al?nd?ktan sonra TCT uyguland?. Demografik veriler, laboratuvar testleri, g???s radyogram? sonu?lar? ve BCG a??lama durumu standart hasta dosyalar?ndan sa?land?. K?rk hastada QFT-G pozitifti. Elli alt? hastan?n TCT ind?rasyonu 5 mm?nin, 28 hastan?n 10 mm?nin ?zerindeydi. Altm?? bir hastada BCG skar? vard?. TCT ve QFT-G aras?nda anlaml? istatistiksel korelasyon saptand? (p< 0.05). BCG a??s?z subgrupta TCT 8 (%29) hastada pozitif, QFT-G 11 (%39) hastada pozitifti. Yirmi bir a??s?z hastada her iki test sonu?lar?ndaki uyum %82 κ= 0.61, p= 0.001 idi. BCG a??s?z hemodiyaliz hastalar?nda LTB? i?in TCT ve QFT-G test aras?ndaki uyum iyi iken, a??l? hastalardaki uyum k?t? olarak bulundu. BCG a??lamas? ?lkemizde yayg?n olarak kullan?ld???ndan QFT-G test, LTB? ??phe edilen hemodiyaliz hastalar?nda TCT?den daha kullan??l? bir test olabilir.
Anahtar Kelimeler: Hemodiyaliz hastalar?, latent t?berk?loz infeksiyonu, QuantiFERON-TB Gold test, t?berk?loz cilt testi.
SUMMARY
QuantiFERON-TB Gold test for screening latent tuberculosis infection in hemodialysis patients
Hayriye
SAYARLIO?LU1, Mustafa G?L2, Canan EREN DA?LI3,
Ekrem DO?AN1, Murat ?AH?N4,
Mehmet Ali U?AR4, Nurhan K?KSAL3, Mehmet SAYARLIO?LU4,
M?mtaz Kerim TAHTA5
1 Division of Nephrology, Department of Internal Medicine, Faculty of Medicine, Sutcu Imam University,
Kahramanmaras, Turkey,
2 Department of Microbiology, Faculty of Medicine, Kahramanmaras Sutcu Imam University, Kahramanmara?,
Turkey,
3 Department of Chest Diseases,Faculty of Medicine, Kahramanmaras Sutcu Imam University, Kahramanmara?,
Turkey,
4 Department of Internal Medicine, Faculty of Medicine, Kahramanmaras Sutcu Imam University,
Kahramanmara?, Turkey,
5 Clinic of Internal Medicine, Kahramanmaras State Hospital, Kahramanmaras, Turkey.
Hemodialysis patients are at increased risk of latent tuberculosis infection (LTBI) compared with the general population. QuantiFERON-TB Gold (QFT-G) for LTBI detection is more promising than tuberculin skin test (TST) in hemodialysis patients. The aim of this study is to determine whether the QFT-G is more sensitive than the TST in hemodialysis patients in LTBI. Eighty nine hemodialysis patients were evaluated for latent tuberculosis infection with the TST and QFT-G. Blood was obtained for QFT-G, and then TST was administered to all patients. Demographic information, laboratory tests, chest radiography results and BCG vaccination status were collected on standardized patient medical files. Forty patients had positive QFT-G results. 56 patients had TST induration above 5 mm, 28 patients above 10 mm. 61 patients had BCG vaccination scar. Statistically significant correlation was detected between TST and QFT-G (p< 0.05). In the BCG non-vaccinated subgroup, TST was positive in 8 (29%) patients and the QFT-G was positive in 11 (39%). Among the 21 non vaccinated patients with results for both tests, the concordance between the TST and QFT-G was 82%, κ= 0.61, p= 0.001. We found good agreement between the TST and QFT-G test for LTBI in non vaccinated hemodialysis patients, whereas we found poor agreement in vaccinated patients. Because BCG vaccination is widely used in our country, the QFT-G test might be more useful for the diagnosis of LTBI than TST in hemodialysis patients who are suspected to have LTBI.
Key Words: Hemodialysis patient, latent tuberculosis infection, QuantiFERON-TB Gold test, tuberculin skin test.
Tuberculosis (TB) still remains to be a major health problem all around the world. Tuberculosis can present as either active or latent. Early identification of latent tuberculosis requires suitable screening guidelines and aids the close observation and follow-up of these patients, however there is no gold standard test for that (1).
End stage renal disease (ESRD) patients are at increased risk of latent tuberculosis infection (LTBI) compared to the general population. LTBI is more likely to progress to TB infection in ESRD. Investigations from several countries have shown that the increased risk of TB among patients on long-term dialysis is 6.9 to 52.5 times higher than the rate in the general population (2). Although guidelines recommend screening these patients for LTBI, the tuberculin skin test (TST) is believed to be insensitive in ESRD patients and TST negativity rate is high. False-negative TST of ESRD patients might be due to the immunocompromised condition (3). In addition, the TST might be falsely positive in persons with a history of previous nontuberculous mycobacterium (NTM) infection or vaccination with Bacillus Calmette-Guerin (BCG) (4).
The QuantiFERON-TB Gold (QFT-G) test measures antigen-specific IFN-γ secretion by peripheral blood CD4+ T lymphocytes in response to in vitro stimulation with ESAT-6, CFP-10, and TB7.7 peptides (5). IFN-γ assay is more promising than TST for LTBI detection in ESRD patients (6). Screening for TB infection should be performed on persons at high risk for infection or progression to active disease especially in immunocompromised condition such as ESRD.
As expected, there is an increased risk of TB among hemodialysis (HD) patients in developing countries (2). Turkey is one of the countries where the disease is endemic (7). LTBI is 52.5 times more likely to be reactivated in patients with renal failure compared with the general population, so screening is necessary (8). It has also been noted that annual TST plus a routine chest radiograph improves detection of tuberculosis infection (9). The aim of this prospective study was to determine whether the QFT-G is more sensitive than the TST in HD patients in LTBI.
MATERIALS and METHODS
Eighty nine HD patients (52 males and 37 females) were recruited into the study (Figure 1). Mean age was 54.6 ? 14.9 years. These patients had been on HD treatment for at least 3 months. Blood was obtained from all patients for the QFT-G test before HD, and then the TST was administered. Demographic information, laboratory tests, chest radiography results and BCG vaccination status were recorded. None of the patients had any active infection, ongoing connective tissue disease, or immune disorders. The etiologies of primary renal disease of the participants were as follows: chronic glomerulonephritis (4), diabetic nephropathy (27), hypertensive nephropathy (16), polycystic kidney disease (9), chronic pyelonephritis, nephrolithiasis (5), amyloidosis (1), others (5), and unknown etiology (22). The study protocol was approved by the local Hospital Ethics Committee and all the participants provided informed consent.
The patients received tuberculin test by using the Mantoux technique with 0.1 mL (5 tuberculin units) of purified protein derivative intradermally injected into the volar surface of the forearm that did not have the arteriovenous shunt. The investigator read the result of the TST 48-72 hour later. Positivity was defined as an induration diameter >10 mm. At least 5 mm of induration following skin testing together with a chest radiography indicating previous infection was defined as latent tuberculosis infection (10).
QuantiFERON-TB Gold
QFT-G detects IFN-γ production when whole blood is incubated with purified mycobacterial antigens. QFT-G test is an in vitro diagnostic aid that measures a component of cell-mediated immune reactivity to Mycobacterium tuberculosis. 5 mL of whole blood was obtained for the IFN-γ assay. QuantiFERON-TB GOLD test (Cellestis Ltd. Carnegie, Victoria, Australia) was used for the IFN-γ assay. The IFN-γ assay was performed in two stages according to the manufacturer?s instructions and the concentration of IFN-γ was determined using the assay kit, according to the manufacturer?s instructions. One milliliter of whole blood was drawn in each of three separate test tubes. The three tubes were incubated for 16-24 hour at 37?C. Following incubation, the tubes were centrifuged and plasma was removed from each tube. IFN-γ was measured by ELISA according to the manufacturer?s instructions. Samples with ≥ 0.35 IU/mL IFN-γ following stimulation with M. tuberculosis-specific antigens were considered positive, while samples < 0.35 IU/mL were considered negative. The QFT-G test result was considered indeterminate if the concentration of IFN-γ was < 0.35 IU/mL for TB antigens and < 0.5 IU/mL for the positive control.
Statistical Analysis
Descriptive statistics include mean ? standard deviations for continuous variables and frequencies and proportions for categorical variables. Chi-square test was used to compare laboratory tests. Continuous variables were compared by Student?s t test. A value of p< 0.05 was considered significant. Concordance between TST and QFT-G was evaluated using agreement and kappa statistics. The strength of this agreement was examined using Cohen?s kappa (κ), with k value > 0.75 representing excellent agreement beyond chance, 0.40 to 0.75 fair to good agreement, and < 0.40 poor agreement.
RESULTS
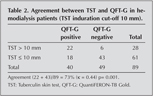
The demographic and clinical characteristics of the patients are shown in Table 1. Forty patients (45%) had positive QFT-G results. 56 patients had TST induration above 5 mm, 28 patients had above 10 mm. Distribution of the patients according to TST and QFT-G positivity are illustrated in Figure 1. Among the 89 patients with results for both tests, the concordance between the TST and the QFT-G was 73%, with a kappa value of 0.44 (TST induration above 10 mm) (Table 2).
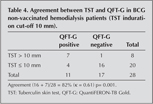
Sixty one (68.5%) patients had been previously BCG vaccinated. In the BCG-vaccinated subgroup, the TST was positive in 20 (33%) patients and the QFT-G was positive in 29 (48%). Among the 61 vaccinated patients with results for both tests, the concordance between the TST and the QFT-G was 69%, with a k value of 0.37, p= 0.003 (TST induration above 10 mm) (Table 3). In the non-vaccinated subgroup, the TST was positive in 8 patients (29%) and the QFT-G was positive in 11 (39%). Among the 28 non vaccinated patients with results for both tests, the concordance between the TST and the QFT-G was 82%, with a k value of 0.61, p= 0.001 (TST induration above 10 mm) (Table 4). k values for BCG vaccinated and non-vaccinated were 0.37, 0.61 respectively.
No significant difference was detected when positivity of QFT-G was compared in patients according to the underlying diseases. TST results among the diabetic patient population were as follows: >10 mm in 8 patients and 13 patients had positive QFT-G (28.6%, p= 0.44 vs. 31.5%, p= 0.51 respectively). No evidence was found when the patients were evaluated with respect to TB infection.
Six patients had TST > 10 mm while their QFT-G was negative, whereas 18 patients had TST < 10 mm while their QFT-G was positive.
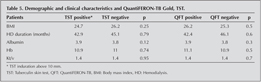
There were no significant differences in terms of mean age, gender, body mass index (BMI), serum albumin levels, kt/v, hemoglobin, between TST positive and negative, and QFT-G positive and negative groups. BMI and kt/v were also significantly different between females and males (kt/v 1.5 vs 1.3 p=0.01, BMI 27.4 vs 24.5 p= 0.02, respectively). Median blood lymphocyte count of the patients was 2228 ? 976 (1000-7000) (Table 5).
DISCUSSION
The TST has been employed for the diagnosis of TB infection. However, sensitivity of the TST is low in HD patient because of the cross-reactivity of TST with the BCG vaccine k which has been used widely in Turkey- and atypical or NTM infections. Thus, its usefulness is limited. Moreover, TST can be false negative because of biological problems, or technical problems related with the TST administration, TST material, or reading. In addition, ESRD is known to be a risk factor for false TST negativity. Sensitivity of ESRD patients decline in parallel with decreasing cellular immune system function. Due to these reasons TST is not a good marker for diagnosis in HD patients. Therefore, this indicates that additional clinical-biochemical test is needed in HD patients other than TST. Standing from this need, we simultaneously compared the performance of QFT-G with TST for detection of LTBI in HD patients in a TB endemic area.
Forty persons (45%) had positive QFT-G results. 28 patients had TST induration above 10 mm. Patients? laboratory parameters were compared with QFT-G and TST positivity. Based on the literature, our study accepted TST > 10 mm as LTBI with respect to TB risk factors (10). QFT-G positivity was 45%, TST positivity was 31.5% (Table 2). The concordance between the TST and the QFT-G was 73%, with a k value of 0.44 p< 0.001. In the present study, we have shown that there is moderate agreement between the TST and QFT-G assay results when TST induration is above 10 mm. Patients with a medical history or family history of TB had positive QFT-G values and no statistical difference was detected between patients with or without exposure to TB.
There are two important causes of false positive tests, atypical or NTM and BCG vaccination. Estimates of the frequency of false positive TST due to NTM range from 1 to 5% of positive TST (11). BCG vaccination is a well known but frequently misunderstood cause of false positive tuberculin reactions. Vaccination after the first year of life causes a stronger and longer lasting effect. In Turkey, BCG vaccination is performed in the first year of life and also at the beginning of the primary school. In our study, BCG vaccinated patients had a low agreement (k= 0.36) between TST and QFT-G (Table 3). Among the 28 non-vaccinated patients with results for both tests, the concordance between TST and QFT-G was 82%, with a k value of 0.61 (Table 4). The discrepancy in the results may be explained by false-positive TST results due to BCG vaccination. Studies carried out in healthy populations showed a strong correlation between TST and QFT-G as a result of LTBI scanning in countries in which vaccination was not practiced (12). Diel et al. detected that there was a good agreement between TST and QFT-G among patients without vaccination (13). Triverio et al. and Lee et al. had studies including vaccinated patients showing poor correlation between TST and QFT-G (14,15). The possible reason of poor correlation among those with BCG is the fact that TST could give false positive result depending on BCG. However, unlike TST, QFT-G was effective in detecting T-cell responses against TB in immunocompromised individuals. It can be said that in our study QFT-G is more reliable than TST in patients without previous BCG vaccination.
A study done by Cengiz has revealed that tuberculosis incidence is 23.6% among HD patients in Turkey where tuberculosis is seen relatively more frequent (16). Our study has found high prevalence of LTBI in HD patients (31% 28/89). The major limitation of this study, as in all studies of this nature, is the lack of a gold standard for the diagnosis of LTBI. High prevalence of QFT-G positivity may have several explanations such as endemic area for TB, their frequent hospital contacts, their old age, and uremic immunological defect. HD patients might have a higher rate of previous tuberculosis infection (17). However, when patients over or equal to and under 65 years of age were compared, there were no significant differences with respect to both TST and QFT-G positivity (p= 0.80 vs 0.65, respectively). In our study, there wasn?t any TB contact or TB history that can explain the high prevalence of LTBI. TB history and contact tracing were rare as the patients could conceal this illness because of social pressure, even if one of their family members had TB.
The prevalence of indeterminate QFT-G results among dialysis patients ranged from 2 to 24%. There is not enough data about which factors influence indeterminate results. Lower CD4 + count, lymphopenia, older age, female sex, diabetes mellitus, cancer chemotherapy and immunosuppressive treatment have been previously reported to be associated with indeterminate results (18,19). There was no indeterminate result in our study including patient?s age. None of them had cancer and none was receiving chemotherapy or immunosuppressive treatment.
While TST-positive and QFT-G test-negative results in immunocompromised patients were attributed to BCG vaccination or NTM infection, TST-negative and QFT-G test-positive results were attributed to immunocompromised patients with a past history of TB infection. No evidence was found to consider active infection in QFT-G positive patients. Chest radiography was taken to exclude pulmonary TB. On the other hand, we performed physical examination especially for lymphadenopathy and mass, asked the history of weight loss for excluding extrapulmonary TB (10). There was no sign of extrapulmonary TB.
When QFT-G positive and negative patients were compared in terms of their BMI, HD duration, blood albumin, ktv, and hg, there were no statistically significant differences between them (Table 5). QFT-G positivity was significantly more frequent in males compared to females. BMI and kt/v were also significantly different between females and males.
In conclusion, we found good agreement between the TST and QFT-G test for LTBI in non vaccinated HD patients, whereas we found poor agreement in vaccinated patients. TB history and contact tracing were rare as the patients could conceal this illness because of social pressure, even if one of their family members had TB. Because vaccination is widely used in our country, the QFT-G test might be more useful for the diagnosis of LTBI than TST in HD patients. Further prospective studies are required to determine whether the QFT-G is more sensitive than the TST in HD patients in LTBI.
ACKNOWLEDGEMENT
This study was supported by the research foundation of Kahramanmaras Sutcu Imam University (2008/1-39M).
CONFLICT of INTEREST
None declared.
REFERENCES
- American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000; 161: (Suppl 1): S221-S247. [Tam Metin] [PDF]
- Hussein MM, Mooij MJ, Roujouleh H. Tuberculosis and chronic renal disease. Semin Dial 2003; 16: 38-44. [?zet]
- Smirnoff M, Patt C, Seckler B, Adler JJ. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest 1998; 113: 25-7. [?zet]
- Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille-Calmette-Guerin vaccination on tuberculin skin test measurements. Thorax 2002; 57: 804-9. [?zet]
- Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004; 4: 761-76. [?zet]
- Winthrop KL, Nyendak M, Calvet H, Oh P, Lo M, Swarbrick G, et al. Interferon-gamma release assays for diagnosing mycobacterium tuberculosis infection in renal dialysis patients. Clin J Am Soc Nephrol 2008; 3: 1357-63. [?zet] [PDF]
- ?zkara ?, Akta? Z, ?zkan S, Ecevit H. T?rkiye?de T?berk?lozun Kontrol? ??in Ba?vuru Kitab?. Ankara: Sa?l?k Bakanl??? Verem Sava?? Daire Ba?kanl???, 2003: 9-10.
- Passalent L, Khan K, Richardson R, Wang J, Dedier H, Gardam M. Detecting latent tuberculosis infection in hemodialysis patients: a head-to-head comparison of the T-SPOT. TB test, tuberculin skin test, and an expert physician panel. Clin J Am Soc Nephrol 2006;2:68-73. [?zet] [Tam Metin] [PDF]
- Wauters A, Peetermans WE, Van den Brande P, De Moor B, Evenepoel P, Keuleers H, et al. The value of tuberculin skin testing in haemodialysis patients. Nephrol Dial Transplant 2004;19: 433-8. [?zet] [Tam Metin] [PDF]
- Choudhury D, Salazar CL. Preventive health care in chronic kidney disease and end-stage renal disease. Nat Clin Pract Nephrol 2008; 4: 194-206. [?zet] [Tam Metin] [PDF]
- Farhat M, Greenaway C, Pai M, Menzies D. False positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria?. Int J Tuber Lung Dis 2006; 10: 1192-204. [?zet]
- Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004; 170: 65-9. [?zet] [Tam Metin] [PDF]
- Diel R, Nienhaus A, Lange C, Meywald-Walter K, Forssbohm M, Schaberg T. Tuberculosis contact investigation with a new, specific blood test in a low-incidence population containing a high proportion of BCG-vaccinated persons. Respir Res 2006; 7: 77. [?zet] [Tam Metin] [PDF]
- Triverio PA, Bridevaux PO, Roux-Lombard P, Niksic L, Rochat T, Martin PY, et al. Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transplant 2009; 24: 1952-6. [?zet] [Tam Metin] [PDF]
- Lee SS, Chou KJ, Su IJ, Chen YS, Fang HC, Huang TS, et al. High prevalence of latent tuberculosis infection in patients in end-stage renal disease on hemodialysis: comparison of QuantiFERON-TB GOLD, ELISPOT, and tuberculin skin test. Infection 2009; 37: 96-102. [?zet]
- Cengiz K. Increased incidence of tuberculosis in patients undergoing hemodialysis. Nephron 1996; 73: 421-4. [?zet]
- Rutsky EA, Rostand SG. Mycobacteriosis in patients with chronic renal failure. Arch Intern Med 1980; 140: 57-61. [?zet]
- Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J 2007; 30: 945-50. [?zet] [Tam Metin] [PDF]
- IInoue T, Nakamura T, Katsuma A, Masumoto S, Minami E, Katagiri D,et al. The value of QuantiFERON TB-Gold in the diagnosis of tuberculosis among dialysis patients. Nephrol Dial Transplant 2009; 24: 2252-7. [?zet] [Tam Metin] [PDF]
Yaz??ma Adresi (Address for Correspondence):
Dr. Hayriye SAYARLIO?LU,
Kahramanmara? S?t?? ?mam ?niversitesi T?p Fak?ltesi
Ara?t?rma Hastanesi ?? Hastal?klar? Anabilm Dal?,
Nefroloji Bilim Dal?,
KAHRAMANMARA? - TURKEY
e-mail: hayriyesayarlioglu@yahoo.com