Akci?er
kanserinin evrelemesinde PET/BT ile kombine edilen
transbron?iyal i?ne aspirasyon
biyopsisinin yeri
?ermin
B?REK??1, Osman ELBEK1, Nazan BAYRAM1, Nevin
UYSAL1, Kemal BAKIR2,
Sabri Z?NC?RKESER3, Maruf ?ANLI4, ?ner D?KENSOY1,
Erhan EK?NC?1
1 Gaziantep ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Gaziantep,
2 Gaziantep ?niversitesi T?p Fak?ltesi, Patoloji Anabilim Dal?, Gaziantep,
3 Gaziantep ?niversitesi T?p Fak?ltesi, N?kleer T?p Anabilim Dal?, Gaziantep,
4 Gaziantep ?niversitesi T?p Fak?ltesi, G???s Cerrahisi Anabilim Dal?, Gaziantep.
?ZET
Akci?er kanserinin evrelemesinde PET/BT ile kombine edilen transbron?iyal i?ne aspirasyon biyopsisinin yeri
Bu ?al??mada pozitron emisyon tomografi/bilgisayarl? tomografi (PET/BT) incelemesi rehber al?narak uygulanan transbron?iyal i?ne aspirasyonu (TB?A) i?leminin akci?er kanseri evrelemesindeki yerini saptamay? ama?lad?k. Mart 2006-Mart 2008 tarihleri aras?nda prospektif olarak, Gaziantep ?niversitesi T?p Fak?ltesi G???s Hastal?klar? Klini?ine ba?vuran, akci?er kanseri ?n tan?s? d???n?len, toraks BT'de 1 cm'den b?y?k lenfadenopati saptanan ve PET/BT tetkikinde SUVmax ≥ 2.5 olan 25 hasta ?al??maya dahil edildi. Toplam 43 lenf nodu istasyonundan TB?A ?rneklemesi yap?ld?. Mediasten de?erlendirmesinde mediastinoskopi sonucu alt?n standart olarak kabul edildi. TB?A y?nteminin mediastinal evrelemedeki duyarl?l?k, ?zg?ll?k, pozitif prediktif, negatif prediktif ve do?ruluk de?erleri s?ras?yla; %67, %100, %100, %76 ve %84 olarak bulundu. Yeterli TB?A ?rneklemesi yap?lan 19 olgunun; 13 (%69)'?nde? ba?lang?? klinik evresi TB?A sonras? gerilemi?, mediastinoskopi sonras? 17 (%89)'sinin? evrelemesinin do?ru olarak yap?ld??? saptanm??t?r. Yirmi be? olgunun 6 (%24)'s?nda? TB?A ile mediastinoskopi ihtiyac? ortadan kalkm??t?r. Pozitif TB?A sonucunu ?ng?rebilecek klinik fakt?rlerden sadece PET SUVmax de?erinin TB?A pozitifli?ini etkiledi?i saptand? [OR= 1.27 (1.004-1.610), p= 0.046]. PET SUVmax ≥ 5'in TB?A pozitifli?ini yakla??k 11 kat art?rd??? g?sterilmi?tir [OR= 10.68 (1.91-59.62), p< 0.01]. Sonu? olarak; akci?er kanseri olgular?n?n do?ru olarak evrelendirilmesinde mediastinoskopiye k?yasla daha az invaziv ve komplikasyonu daha az bir i?lem olan TB?A'n?n kullan?labilece?i, PET/BT tetkiki ile birlikte uygulanmas?n?n TB?A'n?n duyarl?l???n? art?rd???, ?zellikle SUVmax ≥ 5 olan lenf nodlar?nda TB?A pozitifli?inin anlaml? oranda y?kseldi?i ve TB?A'n?n mediastinoskopi ihtiyac?n? azaltt??? sonucuna var?lm??t?r.
Anahtar Kelimeler: Akci?er kanseri, evreleme, TB?A/BT, mediastinoskopi.
SUMMARY
Combined transbronchial needle aspiration and PET/CT for mediastinal staging of lung cancer
?ermin
B?REK??1, Osman ELBEK1, Nazan BAYRAM1, Nevin
UYSAL1, Kemal BAKIR2,
Sabri Z?NC?RKESER3, Maruf ?ANLI4, ?ner D?KENSOY1,
Erhan EK?NC?1
1 Department of Chest Diseases, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey,????????
2 Department of Pathology, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey,
3 Department of Nuclear Medicine, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey,
4 Department of Chest Surgery, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey.
In this study, we aimed to evaluate the performance of transbronchial needle aspiration (TBNA) combined with positron emission tomography/computed tomography (PET/CT) for the staging of lung cancer. Twenty-five patients having lymphadenopathies greater than 1 cm on thorax CT and maximum standardized uptake value (SUVmax) ≥ 2.5 on PET/CT were included in this prospective study performed between March 2006 and March 2008. Forty-three lymphnode stations were sampled by using TBNA. Surgical histology, as confirmed by mediastinoscopy, was accepted as the "gold standard". The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of combined TBNA and PET/CT for correct lymph node staging were 67%, 100%, 100%, 76% and 84%; respectively. The initial clinical staging was downstaged after TBNA in 13/19 (69%) patients with adequate TBNA samples, whereas staging was correct in 17/19 (89%) patients assessed by combined TBNA and PET/CT. Staging was completed by TBNA, without mediastinoscopy, in 6/25 (24%) patients. Among the clinical factors that were assessed, only the PET SUVmax was associated with positive TBNA results [odds ratio (OR) 1.27, 95% CI 1.004-1.61, p= 0.046]. A PET SUVmax ≥ 5 was eleven times more likely in patients with positive TBNA results [OR 10.68, 95% CI 1.91-59.62, p< 0.01]. In conclusion, the combination of TBNA with PET/CT increased the sensitivity of TBNA. Combined TBNA and PET/CT may also allow adequate mediastinal staging of lung cancer in most patients with enlarged lymph nodes, and reduce the need for mediastinoscopy. The SUVmax cut off point for a positive TBNA result was ≥ 5.
Key Words: Lung cancer, staging, TBNA, PET/CT, mediastinoscopy.
The appropriate treatment of patients with lung cancer depends on accurate staging. If there are no distant metastases, the staging of mediastinal lymph nodes is critical. Resection is not considered for lung cancer patients with mediastinal lymph node involvement (1).
Various diagnostic tools are used for pre-operative staging of lung cancer. Positron emission tomography (PET) has shown substantial promise during the past decade, as a tool for non-invasive pre-operative staging of lung cancer (2). Integrated PET and computed tomography (PET/CT) has proven to be more accurate than CT or PET alone for pre-operative mediastinal staging (3,4). However, the frequency of false positive results with integrated PET/CT has not been determined; therefore confirmation by tissue histology is often required (5).
Mediastinoscopy is still the "gold standard" method for staging of mediastinal lymph nodes (6). However, mediastinoscopy is performed under general anesthesia, resulting in higher costs and increased morbidity and mortality as compared with fiberoptic bronchoscopy. In order to avoid these problems, transbronchial needle aspiration (TBNA) in conjunction with fiberoptic bronchoscopy was developed in 1983, and diagnostic yield improved after lymph node mapping was completed (7,8). There have only been a few studies investigating the utility of TBNA and PET/CT for mediastinal staging of lung cancer (9). The aim of this study was to determine whether combined TBNA and PET/CT increases the sensitivity of TBNA, and has the potential to allow adequate mediastinal staging of lung cancer and reduce the need for mediastinoscopy.
MATERIALS and METHODS
A prospective study was performed on 25 patients having lymphadenopathies greater than 1 cm on thorax CT and maximum standardized uptake value (SUVmax) ≥ 2.5 on PET/CT with suspected lung cancer at the? Pulmonary Diseases Department of Gaziantep University, between March 2006 and March 2008. Forty-three lymph node stations were sampled by using TBNA. The study was approved by the ethics committee of the hospital.
Subjects
Patients were included in this prospective study if they had no evidence of distant metastases as assessed by CT and PET/CT. All patients were considered to be candidates for curative thoracic surgery.
PET-CT Scanning
Whole-body PET-CT imaging was performed on a Siemens Biograph 2 PET-CT system. Whole-body acquisition was performed one hour after intravenous administration of 18F-2-fluoro-2-deoxyglucose (FDG) (11-16 mCi) and images were obtained from the vertex to the upper thigh region. High-quality images were acquired and semi-quantitative measurements of glucose metabolism were obtained. All patients fasted for at least four hours before imaging and fasting blood glucose levels were within the normal range. None of the patients received insulin to bring blood glucose levels back to normal. The SUVs of the hilar lymph nodes and mediastinal lymph nodes were determined from transverse views by the nuclear medicine physician. Coronal-sagittal images and their correlation with CT were used whenever the exact location was uncertain. Regions of interest (ROI) were drawn on the images and SUVmax was semi-quantitatively defined as the regional tissue concentration of radioactivity normalized for the injected dose and body weight of the patient. The PET-CT scan was considered to be positive in the mediastinum and hilar area that was separate from the primary mass, if the SUVmax in patients with suspected lymph node metastases was > 2.5.
Bronchoscopic Procedures
To determine the location of lymph node aspiration, Wang's lymph node mapping system was used after PET/CT scanning (8). Patients received midazolam as premedication for bronchoscopy, with 0.1% pantocaine as the local anesthetic agent. TBNA was performed from lymph node stations localized by PET/CT, using an Olympus BF Type P20D bronchoscope with a 22G cytology needle and sampling from endobronchial or endotracheal locations, as previously described by Wang (10,11). Cytological samples were obtained from one to three sites, with four passes for each patient. In patients with more than one lymph node site to be sampled with TBNA, lymph nodes were sampled in the following order, starting from N3 to N1.? In order to avoid cytological contamination TBNA was performed before other bronchoscopic procedures. All procedures were performed by one bronchoscopist. Transbronchial needle aspirates were smeared onto four to six slides, immediately fixed in %95 alcohol and transported to the pathology laboratory. All specimens were evaluated by the same pathologist. TBNA specimens were accepted as adequate if numerous benign lymphoid cells were present, indicating that the sample had been obtained from a lymph node. Cytological analysis was considered positive only when a sufficient number of cells that were definitely malignant were observed. All other results were considered negative.
Mediastinoscopy
Surgical histology, as confirmed by mediastinoscopy, was considered the "gold standard". The decision to perform mediastinoscopy or curative surgical resection with complete lymph node dissection was taken at the weekly multidisciplinary meeting of the pulmonologist, radiologist, thoracic surgeon, pathologist, oncologist, and nuclear medicine physician. Mediastinoscopy was performed in all patients with negative results for TBNA of lymph nodes.
Statistical Analysis
Surgical histology, as confirmed by mediastinoscopy, was considered the "gold standard". The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for prediction of lymph node staging using PET/CT combined with TBNA were calculated.
Descriptive statistics were expressed as mean ? standard deviation (SD), interquartile range (IQR) or percentages depending on the type of data. The clinical factors that might be associated with a positive TBNA result, including lymph node location, PET SUVmax, late PET SUV, TBNA results, and tumor cell type, were assessed using a logistic regression model. The association between the clinical factors and the positive TBNA results were expressed as odds ratios (OR) with 95% confidence intervals (CI). Statistical analyses were performed using SPSS 13.0 for Windows and p values < 0.05 were deemed to be statistically significant.
RESULTS
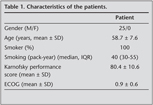
Twenty-five patients having lymphadenopathies greater than 1 cm on thorax CT and SUVmax ≥ 2.5 on PET/CT were included in this study. The characteristics of these patients are summarized in Table 1.
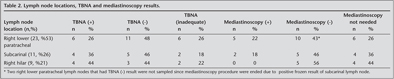
All 43 enlarged mediastinal lymph nodes were sampled. The sites of the sampled lymph nodes were right paratracheal 23 (53%), subcarinal 11 (26%), and right hilar 9 (21%). Among these 43 lymph node stations that were sampled by TBNA, 10 TBNA samples (23%) from six patients were inadequate as no lymphocytes were found, and 33 TBNA samples (77%) from 19 patients were adequate (Table 2).
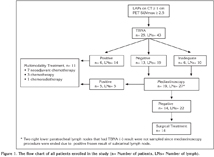
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of TBNA combined with PET/CT for correct diagnosis of lymph node staging among the 43 sampled lymph node stations were 67%, 100%, 100%, 76% and 84%; respectively. The initial clinical staging was changed after TBNA in 13/19 (69%) patients, whereas the staging was correct with TBNA and PET/CT in 17/19 (89%) patients with adequate TBNA samples. Furthermore, the staging was correct with TBNA and PET/CT in 17 of the 25 patients (68%) enrolled in the study. Staging was completed by TBNA, without mediastinoscopy, in 6/25 (24%) patients. Minor bleeding after the TBNA procedure was observed in 7 (28%) patients. Details of the procedures performed on all patients enrolled in the study are shown in Figure 1.
Logistic regression analysis showed that among the clinical factors assessed, only the PET SUVmax was associated with positive TBNA results [OR 1.27, 95% CI 1.004-1.610, p= 0.046]. A PET SUVmax ≥ 5 was eleven times more likely to be associated with positive TBNA results [OR 10.68, 95% CI 1.91-59.62, p< 0.01] (Table 3).
The final diagnoses and treatment for the 25 patients were as follows: 23 patients (92%) were diagnosed with non-small cell lung carcinoma (NSCLC) and two (8%) were diagnosed with small cell lung carcinoma (SCLC); 14 patients (56%) underwent curative resection, seven (28%) received neoadjuvant chemoradiotherapy, three (12%) received chemotherapy and one (4%) received chemoradiotherapy.
DISCUSSION
The objective of this investigation was to show that the combination of TBNA and PET/CT increases sensitivity of TBNA, and has the potential to allow adequate mediastinal staging of lung cancer in most patients with enlarged lymph nodes.
Non-invasive lung cancer staging was substantially improved with the use of PET. Many prospective series and six meta-analyses have reported the superiority of PET versus CT for primary staging of the mediastinal LN's (12,13,14,15,16). Current fusion scanners now acquire superimposed PET and CT images. Studies show increased diagnostic accuracy of integrated PET/CT relative to PET scan alone (13). Cerfolio reported that when evaluating N2 disease, the PET-CT scan was more accurate than PET alone (96% versus 93%) (4). A study by Kelly et al. concluded that adding a PET scan to a CT scan in the clinical staging of NSCLC patients would improve the identification of N2 disease in the preoperative period (sensitivity, specificity, and accuracy rates were improved; 70%, 98%, and 91%; respectively) (17). However, PET/CT? is not infallible as certain non neoplastic processes, including infectious, granulomatous, and other inflammatory diseases, may result in positive PET findings. This is especially the case in populations with a high prevalence of tuberculosis, or a high rate of inorganic dust exposure. All suspicious and decisive areas observed on PET should be biopsied so that patients are not precluded from having potentially curative surgery (18).
TBNA is a minimally invasive approach that provides histological or cytological confirmation of nodal tumor involvement. TBNA has been shown to be a valuable tool for diagnosing and staging bronchogenic carcinoma, and also diagnosing benign mediastinal or hilar lymph nodes, although the yield varies widely from 65% to 80% for bronchogenic carcinoma, 60% to %85 for benign mediastinal or hilar lymph nodes (19,20,21,22,23,24,25,26). Adding TBNA to conventional diagnostic methods (forceps biopsy, bronchial washing, bronchial brushing) increases the diagnostic success in lung cancer (25,26,27). TBNA is usually combined with CT, and in recent years with PET and PET/CT, to increase sensitivity and specificity. In a meta-analysis done by Holty et al. the median sensitivity and specificity of TBNA combined with CT were 36% and 98%; respectively, while the pooled sensitivity was 39%, and the pooled specificity was 99% (28). In a previous study performed in our clinic before the availability of PET/CT, we found that the sensitivity, specificity, posistive predictive value, negative predictive value, and accuracy of TBNA combined with CT in the diagnosis of lung cancer patients was 58%, 100%, 100%, 37% and 66%; respectively (29). There have only been a small number of studies on TBNA combined with PET, and PET/CT.? Bernasconi and colleagues reported that, the sensitivity, specificity, positive predictive value, negative predictive value and accuracy for detecting malignant lymphadenopathy with combined TBNA and PET was 100%, 94%, 100%, 79%, and 95%; respectively (30). Hsu and his colleagues showed that the accuracy and sensitivity of TBNA combined with PET/CT for subcentimetre nodes was 88.9% and 87.5%; respectively. In our study the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of the combination of TBNA with PET/CT for the correct diagnosis of lymph node staging was 67%, 100%, 100%, 76% and 84%, respectively. The 84% accuracy ratio of? is not so high compare to the result of studies without PET/CT, 78% is reported in study of Bilaceroglu et al., 82% is in Uskul et al. 79% in Caglayan et al. 75.8% in Kacar et al., which may be due to difficulties in obtaining adequate lymph node sample (19,25,26,27).
A potential direct clinical impact of the combined approach of using TBNA and PET is the reduced need for mediastinoscopy by diagnosing N2 and N3 involvement at the time of diagnosis (30). Bernasconi and colleagues estimated that based on an approach of combining a negative TBNA and a negative PET, mediastinoscopy could? potentially be obviated in 57% of patients. The percentage of patients who can avoid mediastinoscopy may be further increased with the use of? endobronchial? ultrasound-guided transbronchial needle aspiration (EBUS-TBNA),? or esophageal ultrasound-guided fine needle aspiration,? plus a rapid, on site cytology on adequate sampling (30). EBUS-TBNA is an effective invasive method following PET/CT scanning in the mediastinal staging of potentially operable NSCLC (31). Recently, Hwangho and his colleagues reported that the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EBUS-TBNA for detecting mediastinal metastases was 90%, 100%, 100%, 96.7%, and 97.4%; respectively (31). Similarly, Yasufuku and colleagues showed that, the sensitivity of EBUS-TBNA for diagnosing mediastinal and hilar lymph node staging was 92.3%, specificity was 100%, and diagnostic accuracy was? 98.0% (32). We found that staging was correct with TBNA and PET/CT in 17/19 (89%) patients with adequate TBNA samples. Furthermore, staging was correct with TBNA and PET/CT in 17 of the 25 patients (68%) enrolled in the study. Staging was completed by TBNA, without mediastinoscopy, in 6/25 (24%) patients.
PET is considered positive for lymph nodes if the SUV > 2.5 (33). Recently, Bauwens et al. reported that patients without malignant lymph node involvement showed a lower SUVmax (respective median values of 3.7 and 10.0; p< 0.0001) (34). Furthermore, there is no study showing a relationship between the SUVmax cut off point and a positive TBNA. In our study clinical factors that may affect a positive TBNA result were analysed and we found that only PET SUVmax affected a positive TBNA result [OR= 1.27 (1.004-1.610), p= 0.046]. We found that the cut off point for? SUVmax was? ≥ 5 to have a positive TBNA result? [OR= 10.68 (1.91-59.62), p< 0.01].
Our study had several limitations: the sample size is small, the ratio of inadequate material (10, 23%) is high, it may be better to use EBUS-TBNA, or esophageal ultrasound-guided fine needle aspiration and a rapid, on site cytology for adequate sampling, it may also be better to take SUVmax < 2.5 and include the actual diameter of the lymph node (instead of > 1 cm), to enable calculation of the mean ? SD lymph node size. A further limitation of this study, is the lack of comparison of the group with no PET/CT combination.
In conclusion, a combination of TBNA and PET/CT has the potential to allow adequate mediastinal staging of lung cancer in most patients with enlarged lymph nodes, and can reduce the need for mediastinoscopy. The SUVmax cut off point for a positive TBNA value was found to be ≥ 5.
CONFLICT of INTEREST
None declared.
REFERENCES
- Mountain CF. Revision in the international system for staging lung cancer. Chest 1997; 111: 1710-7. [?zet] [PDF]
- Graeter TP, Hellwig D, Hoffmann K, et al. Mediastinal lymph node staging in suspected lung cancer: comparison of positron emission tomography with F-18-fluorodeoxyglucose and mediastinoscopy. Ann Thorac Surg 2003; 75: 231-5. [?zet] [Tam Metin] [PDF]
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell with integrated positron-emission tomography and computed tomography. N Engl J Med 2003; 348: 2500-7. [?zet] [Tam Metin] [PDF]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET/CT compared with dedicated PET alone for the staging of patients with non-small cell lung cancer. Ann Thorac Surg 2004; 78: 1017-23. [?zet] [Tam Metin] [PDF]
- Eloubeidi MA, Cerfolio RJ, Chen VK, et al. Endoscopic ultrasound-quided fine needle aspiration of mediastinal lymph node in patients with suspected lung? cancer after positron emission tomography and computed tomography scan. Ann Thorac Surg 2005; 79: 263-8. [?zet] [Tam Metin] [PDF]
- Detterbeck FC, DeCamp MM Jr, Kohman LJ, Silvestri GA. Lung cancer. Invasive staging: the quidelines. Chest 2003; 123 (Suppl): S167-75. [?zet] [Tam Metin] [PDF]
- Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis 1983; 127: 344-7. [?zet]
- Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest 1994; 106: 588-93. [PDF]
- Hsu LH, Ko JS, You DL, et al. Transbronchial needle aspiration accurately diagnoses subcentimetre mediastinal and hilar lymph nodes detected by integrated positron emission tomography and computed tomography. Respirology 2007; 12: 848-55. [?zet]
- Wang KP. Transbronchial needle aspiration to obtain histology specimen. J Bronchol 1994; 1: 116-22. [?zet] [PDF]
- Wang KP. Transbronchial needle aspiration: how I do it. J Bronchol 1884; 1: 63-8.
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007; 32: 1-8. [?zet] [Tam Metin] [PDF]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotheraphy for mediastinoscopy-proven stage IIIA-N2 non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006; 24: 3333-9. [?zet] [Tam Metin] [PDF]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med 2003; 139: 879-92. [?zet] [Tam Metin] [PDF]
- Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in non-small cell lung cancer. Ann Thorac Surg 2005; 79: 375-82. [?zet] [Tam Metin] [PDF]
- Tolaza EM, Harpole L, Detterbeck F, McCrory DC. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003; 123 (Suppl): S157-66. [?zet] [Tam Metin] [PDF]
- Kelly RF, Tran T, Holmstrom, et al. Accuracy and cost-effectivenes of [18 F]-2-fluoro-deoxy-D-glucose-positron emission tomography scan in potentially resectable non-small cell lung cancer. Chest 2004; 125: 1413-23. [?zet] [Tam Metin] [PDF]
- Silvestri GA, Tanoue LT, Margolis JB, et al. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest 2003; 123 (Suppl 1): S147-56. [?zet] [Tam Metin] [PDF]
- Bila?ero?lu S, ?a??r?c? U, G?nel ?, et al. Comparison of Rigid and Flexible Transbronchial Needle Aspiration in the Staging of Bronchogenic Carcinoma. Respiration 1998; 65: 441-9. [?zet]
- ?etinkaya E, Y?ld?z P, Kadakal F, et al. Transbronchial needle aspiration in the diagnosis of intrathoracic lymphadenopathy. Respiration 2002; 69: 335-8. [?zet]
- ?etinkaya E, Y?ld?z P, Alt?n S, Y?lmaz V. Diagnostic needle aspiration by wang 22-gauge cytology needle in intrathoracic lymphadenopathy. Chest 2004; 125: 527-31. [?zet] [Tam Metin] [PDF]
- Mehta AC, Kavuru MS, Meeker DP, et al. Transbronchial needle aspiration for histology specimen. Chest 1989; 96: 1228-32. [?zet]
- Wang KP, Brower R, Haponic EF, Siegelman S. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest 1983; 84: 571-6. [?zet] [PDF]
- Harrow EM, Abi-Saleh W, Blum J, et al. The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med 2000; 161: 601-7. [?zet] [Tam Metin] [PDF]
- ?sk?l BT, T?rker H, Meliko?lu A, et al. Value of transbronchial needle aspiration in the diagnosis of endobronchial malignant lesions. Tuberk Toraks 2007; 55: 259-65. [?zet]
- ?a?layan B, Akt?rk UA, Fidan A, et al. Transbronchial needle aspiration in the diagnosis of endobronchial malignant Lesions.? A 3 year Experience. Chest 2005; 128: 704-8. [?zet] [Tam Metin] [PDF]
- Ka?ar N, Tuksavul F, Edipo?lu ?, et al. Effectiveness of transbronchial needle aspiration in the diagnosis of exophytic endobronchial lesions and submucosal/peribronchial diseases of the lung. Lung Cancer 2005; 50: 221-6. [?zet]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005; 60: 949-55. [?zet] [PDF]
- Bayram N, Borekci S, Uyar M, et al. Transbronchial needle aspiration in the diagnosis and staging of lung cancer. Indian J Chest Dis Allied Sci 2008; 50: 273-6. [?zet]
- Bernasconi M, Chhajed PN, Gambazzi F, et al. Combined transbronchial needle aspiration and positron emission tomography for mediastinal staging of NCCLC. Eur Respir J 2006; 27: 889-94. [?zet] [Tam Metin] [PDF]
- Hwangbo B, Kim SK, Lee HS, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009; 135: 1280-7. [?zet] [Tam Metin] [PDF]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006; 130: 710-8. [?zet] [Tam Metin] [PDF]
- Lowe VJ, Hoffman JM, DeLong DM, et al. Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med 1994; 35: 1771-6. [?zet] [PDF]
- Bauwens O, Dusart M, Pierard P, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer 2008; 61: 356-61. [?zet]
Yaz??ma Adresi (Address for Correspondence):
Dr. ?ermin B?REK??,
?stanbul ?niversitesi Cerrahpa?a T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
?STANBUL - TURKEY
e-mail: serminborekci@yahoo.com.tr